Why we are using the clamp technique?
According to the requirements of regulatory agencies [1, 2] pharmacodynamic data on time-actions profiles using the euglycemic glucose clamp procedure should be available for novel or biosimilar insulin preparations (click here to watch the online seminar), including pre-mixed insulins. In addition there is a general agreement among experts that the euglycaemic glucose clamp procedure is the best available method for the assessment of insulin action.
The principle of a glucose clamp experiment consists of antagonizing the blood-glucose lowering effect of a drug (e.g. insulin or oral antidiabetic agent) by means of a variable infusion of glucose, so that blood glucose concentrations are maintained or “clamped” at a pre-defined target level.
In 1979 De Fronzo et al. [3] published the first description of an automated clamp experiment. Our founders Tim Heise [4] and Lutz Heinemann [5] have improved the operationality and the refinement of the automated clamp technique, for which a device called Biostator, developed in the early 1970ies, has predominantly been used until recently.
Automated vs. Manual Glucose Clamps
Clamp studies can be performed manually or by using an automated clamp device. Both techniques require considerable experience to provide reproducible results. The automated clamp technique has the advantage of continuously measuring blood glucose and adjusting glucose infusion rates (GIR) every minute via a feedback algorithm implemented in the device. Thus, in contrast to the manual technique there is no room for investigator-bias in the determination of glucose infusion rates and, most importantly, the adjustments can be done much faster than with the manual technique. This might have particular advantages for fast-acting compounds such as rapid or ultra-rapid insulin analogues which has already been acknowledged by authorities [2]. But also for drugs with a slower onset and a longer duration of action (e.g. basal insulins) it will be of utmost importance to achieve a good clamp quality, i.e. keeping blood glucose levels during the clamp as close as possible to the target level. Technically, each deviation from the blood glucose target indicates that the chosen GIR was not optimal. While short-term and minor deviations are unavoidable, larger deviations over a longer period of time trigger concerns (also at authorities) that GIR-results might not be a true reflection of the drug effect. We recently published an overview on suitable parameters for the assessment of glucose clamp quality [7], but despite vast agreement of clamp experts on the importance of glucose clamp quality [8], this information is unfortunately often missing in published glucose clamp studies.
Next generation automated glucose clamping
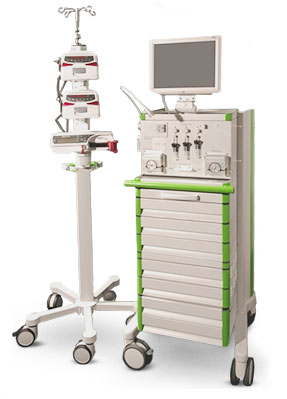
In 2013 Profil introduced ClampArt®, a modern glucose clamp device, to further improve glucose clamp quality [6, 7]. ClampArt® is a CE-certified device meeting harmonised standards (DIN EN 60601-1, DIN EN 60601-1-2, DIN EN ISO 14971, DIN EN 62304, and DIN EN 62366) and uses fully validated, state of the art technology to overcome the limitations of the rather outdated Biostator equipment. The most important improvements consist of the use of modern sensor technology and up-to-date infusion pumps. Thereby, ClampArt® provides more reliable blood glucose results and reduces both glucose and GIR fluctuations. This is mainly achieved through the continuous determination of both glucose and the blood dilution factor so that confounding factors like disturbances in blood flow are taken into account and are automatically corrected. Further improvements, e.g. of the implemented algorithm are in development. Nowadays, the majority of glucose clamps done at Profil Germany are performed with ClampArt®.
Profil offers various clamp methods for versatile study setup
In addition to an excellent device a lot of experience is needed to optimally design, analyse and interpret the results of glucose clamp studies. At Profil Germany a team of scientists, regulatory experts, statisticians and clamp technicians ensures that the expected characteristics of the study drug are adequately captured in the glucose clamp design and the analysed PD-parameters, and, most importantly, that all relevant regulatory guidelines and scientific recommendations are taken into account.
This team-approach is not only used for pharmacodynamic assessments of blood-glucose lowering agents, but also for other investigational objectives the clamp technique can be used for, e.g. the assessment of insulin sensitivity, beta-cell function (hyperglycemic glucose clamps) and hypoglycaemia counter-regulation (hypoglycemic glucose clamps) or metabolic fluxes using stable isotope dilution techniques and sometimes muscle or fat biopsies.




