This is the second part of our series on insulin resistance. If you are uncertain about the basics and the molecular pathways involved, then you should read the first part first. If you want to discuss with our experts and want to see how insulin resistance is measured in mice and humans, then our upcoming free online seminar may be the right thing for you.
Recall the physiological as well as pathological pathways and then enjoy the second part of this blog series on insulin resistance.
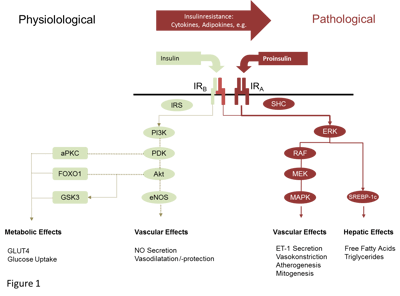
Assessment of insulin resistance
Numerous surrogate measures have been used to quantify insulin resistance, but the accurate assessment of insulin resistance is still a challenge due to the dynamics of glucose homeostasis and the presence of several confounding variables. In addition to insulin and glucose, other biomarkers like adiponectin have been suggested as simple measures to quantify insulin resistance from a single fasting blood sample [51-53]. As already addressed, adiponectin released from the visceral adipose tissue is a linker between visceral adiposity and the development of insulin resistance. The measurement of adiponectin levels is a helpful tool for the assessment of insulin resistance, and monitoring of dynamic changes in adiponectin levels due to therapeutic interventions.
The homeostasis model assessment of insulin resistance (HOMA-IR) is a widely used surrogate to characterise insulin resistance based on a simple measurement of fasting insulin and glucose levels [(fasting insulin concentration x fasting glucose concentration)/22.5] [54]. The quick insulin sensitivity check index (QUICKI) is a log transformation of HOMA to yield directly an insulin sensitivity index [55]. As both indices (HOMA; QUICKI) rely on a fasting blood sampling, and the liver is playing the fundamental role in glucose haemostasis in fasting conditions, they can be assumed to be markers of hepatic insulin sensitivity rather than peripheral insulin sensitivity.
While assessment of insulin resistance under fasting conditions mainly reflects hepatic insulin sensitivity, several technologies have been implemented to address peripheral insulin resistance. The oral glucose tolerance test (OGTT) can be used to calculate the whole body insulin sensitivity index, or to appraise the ratio of glucose and insulin areas under the curve as a simplified measure for insulin resistance in the postprandial stage [56,57]. The frequently sampled intravenous glucose tolerance test (fsIVGTT) establishes insulin resistance by mathematical modelling of insulin and blood glucose levels after intravenous glucose application [58].
A major limitation of the aforementioned HOMA-IR, OGGT-derived indices, and the fsIVGTT is that they rely on intact beta cell physiology, and their reliability become worse in case of declining beta cell function and increasing proinsulin levels [59,60].
The euglycaemic-hyperinsulinaemic clamp is considered to be the gold standard for the assessment of insulin resistance [61-63]. The glucose infusion rate during the steady state phase of a euglycaemic-hyperinsulinaemic clamp is usually achieved after 2-3 hours and after that time, insulin sensitivity is expressed by the average glucose infusion rate over a defined time period (M-value), or the M/I ratio defined as the average glucose infusion to the average plasma insulin concentration during the same period [64]. Since it is assumed that during hyperinsulinaemic conditions hepatic glucose output is widely suppressed, the glucose infusion rate within the hyperinsulinaemic- euglycaemic clamp will mostly reflect peripheral glucose uptake. The additional use of isotope tracers in the clamp investigation enables further quantification of hepatic (endogenous) glucose production and peripheral glucose disposal rates [65]. While the euglycaemic-hyperinsulinaemic clamp arguably is the most standardised and reproducible way to measure insulin resistance, it is expensive, time consuming, and requires experienced investigators. Due to both, its sophisticated methodology and its high expense, conduct of high-quality clamp assessments remains reserved to specialised centres.
Therapeutic Interventions in Insulin Resistance
Whilst lifestyle and weight loss remains the cornerstone of managing IR and T2DM, metformin and thiazolidinediones are the currently available pharmacological options to counter IR. Even they affect hepatic and peripheral IR, their impact is not sustained and they might have significant adverse event profiles. Hence, there is a need to establish new treatments for the intervention in subjects with IR. Certain pharmacological candidates to improve insulin signalling are under preclinical or clinical development.
Insulin receptor activators or insulin mimetics are small molecules that address the insulin receptor or the downstream IRS-proteins. Activating insulin receptor signalling by non-peptide ligands might have the advantage of activating the insulin receptor by a non-peptide ligand, overcoming IR without need for injections [66]. In contrast, insulin receptor potentiators work by prolonging the phosphorylation of the insulin receptor ß-subunit or by antagonising the pathways that inhibit tyrosine kinase activity, or the inhibition of tyrosine phosphatases [67,68]. Protein Tyrosine Phosphatase Inhibitors dephosphorylate the insulin receptor ß-subunit, and inhibition of this process is thought to improve insulin signalling [66,68,69]. Protein kinase C (PKC) inhibitors, Phosphatase and Tensin Homolog (PTEN) inhibitors, Inosol derivates, Inosol Phosohatases Inhibitors, Resveratol, and other molecules have been identified to interfere with intracellular insulin signalling at several trigger points, and are under investigation as potential insulin sensitizing agents [66]. In addition, on-going research on adipokines (e.g. adiponectin; leptin), or adipokine like molecules, should clarify their role as a potential treatments for IR and/or T2DM. A number of drugs in clinical development address obesity and should support weight reduction in obese subjects, which also should evolve pronounced effects on IR.
In conclusion, numerous new approaches are in development to improve insulin sensitivity or insulin signalling in pre-diabetic or diabetic subjects. For a better evaluation of potential risks and benefits of new approaches to address IR, careful pre-clinical and clinical studies should characterize their effects with regard to their metabolic and cardiovascular capabilities.
What comes next?
Having understood the basics of insulin resistance and the underlying molecular pathways as well as the relevance for clinical development and methods to measure insulin resistance is a wonderful foundation to join our online seminar on studying insulin resistance in preclinical and clinical trials. Here you can learn more about how to plan the right experiments and how to optimize study design for a smooth transition from preclinical to clinical trials of a compound. In this online seminar we bring together experts from the preclinical side and clinical trial experts who will discuss this topic in a 45 minute presentation. Register for this free online seminar now, as seats are limited.
References
- Henquin J.C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000; 49:1751-1760.
- Del Prato S., Marchetti P., Bonadonna R.C. Phasic insulin release and metabolic regulation in type 2 diabetes. Diabetes 2002; 51 Suppl 1:S109-S116.
- Polonsky K.S., Given B.D., Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest 1988; 81:442-448.
- Gregg E.W., Cheng Y.J., Cadwell B.L. et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA 2005; 293:1868-1874.
- Kahn S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Int J Obes Relat Metab Disord 2003; 46:3-19.
- Ferrannini E., Mari A. Beta cell function and its relation to insulin action in humans: a critical appraisal. Int J Obes Relat Metab Disord 2004; 47:943-956.
- Howard G., O'Leary D.H., Zaccaro D. et al. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation 1996; 93:1809-1817.
- Westergren H.U., Svedlund S., Momo R.A. et al. Insulin resistance, endothelial function, angiogenic factors and clinical outcome in non-diabetic patients with chest pain without myocardial perfusion defects. Cardiovasc Diabetol 2016; 15:36.
- Bonora E., Formentini G., Calcaterra F. et al. HOMA-Estimated Insulin Resistance Is an Independent Predictor of Cardiovascular Disease in Type 2 Diabetic Subjects: Prospective data from the Verona Diabetes Complications Study. Diabetes Care 2002; 25:1135-1141.
- Yip J., Facchini F.S., Reaven G.M. Resistance to insulin-mediated glucose disposal as a predictor of cardiovascular disease. J Clin Endocrinol Metab 1998; 83:2773-2776.
- Roberts A.C., Porter K.E. Cellular and molecular mechanisms of endothelial dysfunction in diabetes. Diab Vasc Dis Res 2013; 10:472-482.
- Saltiel A.R., Kahn C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001; 414:799-806.
- Taniguchi C.M., Ueki K., Kahn R. Complementary roles of IRS-1 and IRS-2 in the hepatic regulation of metabolism. J Clin Invest 2005; 115:718-727.
- Montagnani M., Quon M.J. Insulin action in vascular endothelium: potential mechanisms linking insulin resistance with hypertension. Diabetes Obes Metab 2000; 2:285-292.
- Kuboki K., Jiang Z.Y., Takahara N. et al. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo : a specific vascular action of insulin. Circulation 2000; 101:676-681.
- Zeng G., Nystrom F.H., Ravichandran L.V. et al. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 2000; 101:1539-1545.
- Loscalzo J. Nitric oxide and vascular disease. N Engl J Med 1995; 333:251-253.
- Loscalzo J. Antiplatelet and antithrombotic effects of organic nitrates. Am J Cardiol 1992; 70:18B-22B.
- Hsueh W.A., Lyon C.J., Quinones M.J. Insulin resistance and the endothelium. Am J Med 2004; 117:109-117.
- Shimomura I., Matsuda M., Hammer R.E., Bashmakov Y., Brown M.S., Goldstein J.L. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell 2000; 6:77-86.
- Montagnani M., Golovchenko I., Kim I. et al. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem 2002; 277:1794-1799.
- Bluher M. Adipokines - removing road blocks to obesity and diabetes therapy. Mol Metab 2014; 3:230-240.
- Zachariah J.P., Hwang S., Hamburg N.M. et al. Circulating Adipokines and Vascular Function: Cross-Sectional Associations in a Community-Based Cohort. Hypertension 2015.
- Yadav A., Kataria M.A., Saini V., Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta 2013; 417:80-84.
- Yamauchi T., Kamon J., Waki H. et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001; 7:941-946.
- Kowalska I., Straczkowski M., Nikolajuk A. et al. Serum visfatin in relation to insulin resistance and markers of hyperandrogenism in lean and obese women with polycystic ovary syndrome. Hum Reprod 2007; 22:1824-1829.
- Dray C., Knauf C., Daviaud D. et al. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab 2008; 8:437-445.
- Cusi K., Maezono K., Osman A. et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest 2000; 105:311-320.
- Eringa E.C., Stehouwer C.D., Roos M.H., Westerhof N., Sipkema P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese Zucker (fa/fa) rats. Am J Physiol Endocrinol Metab 2007; 293:E1134-E1139.
- Jiang Z.Y., Lin Y.W., Clemont A. et al. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest 1999; 104:447-457.
- Potenza M.A., Marasciulo F.L., Chieppa D.M. et al. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol 2005; 289:H813-H822.
- Prior J.O., Quinones M.J., Hernandez-Pampaloni M. et al. Coronary circulatory dysfunction in insulin resistance, impaired glucose tolerance, and type 2 diabetes mellitus. Circulation 2005; 111:2291-2298.
- Weyer C., Hanson R.L., Tataranni P.A., Bogardus C., Pratley R.E. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes 2000; 49:2094-2101.
- Verma S., Yao L., Stewart D.J., Dumont A.S., Anderson T.J., McNeill J.H. Endothelin antagonism uncovers insulin-mediated vasorelaxation in vitro and in vivo. Hypertension 2001; 37:328-333.
- Cardillo C., Nambi S.S., Kilcoyne C.M. et al. Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation 1999; 100:820-825.
- Eringa E.C., Stehouwer C.D., Nieuw Amerongen G.P., Ouwehand L., Westerhof N., Sipkema P. Vasoconstrictor effects of insulin in skeletal muscle arterioles are mediated by ERK1/2 activation in endothelium. Am J Physiol Heart Circ Physiol 2004; 287:H2043-H2048.
- Scotland R., Vallance P., Ahluwalia A. Endothelin alters the reactivity of vasa vasorum: mechanisms and implications for conduit vessel physiology and pathophysiology. Br J Pharmacol 1999; 128:1229-1234.
- Filep J.G., Sirois M.G., Foldes-Filep E. et al. Enhancement by endothelin-1 of microvascular permeability via the activation of ETA receptors. Br J Pharmacol 1993; 109:880-886.
- Bobik A., Grooms A., Millar J.A., Mitchell A., Grinpukel S. Growth factor activity of endothelin on vascular smooth muscle. Am J Physiol 1990; 258:C408-C415.
- Stankova J., Rola-Pleszczynski M., D'Orleans-Juste P. Endothelin 1 and thrombin synergistically stimulate IL-6 mRNA expression and protein production in human umbilical vein endothelial cells. J Cardiovasc Pharmacol 1995; 26 Suppl 3:S505-S507.
- McMillen M.A., Huribal M., Cunningham M.E., Kumar R., Sumpio B.E. Endothelin-1 increases intracellular calcium in human monocytes and causes production of interleukin-6. Crit Care Med 1995; 23:34-40.
- DeFronzo R.A., Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991; 14:173-194.
- Cao W., Ning J., Yang X., Liu Z. Excess exposure to insulin is the primary cause of insulin resistance and its associated atherosclerosis. Curr Mol Pharmacol 2011; 4:154-166.
- Schenk S., Saberi M., Olefsky J.M. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 2008; 118:2992-3002.
- Nolan C.J., Ruderman N.B., Kahn S.E., Pedersen O., Prentki M. Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes 2015; 64:673-686.
- Chess D.J., Stanley W.C. Role of diet and fuel overabundance in the development and progression of heart failure. Cardiovasc Res 2008; 79:269-278.
- Labbe S.M., Grenier-Larouche T., Noll C. et al. Increased myocardial uptake of dietary fatty acids linked to cardiac dysfunction in glucose-intolerant humans. Diabetes 2012; 61:2701-2710.
- Jagasia D., Whiting J.M., Concato J., Pfau S., McNulty P.H. Effect of non-insulin-dependent diabetes mellitus on myocardial insulin responsiveness in patients with ischemic heart disease. Circulation 2001; 103:1734-1739.
- Jankovic D., Winhofer Y., Promintzer-Schifferl M. et al. Effects of insulin therapy on myocardial lipid content and cardiac geometry in patients with type-2 diabetes mellitus. PLoS One 2012; 7:e50077.
- Winhofer Y., Krssak M., Jankovic D. et al. Short-term hyperinsulinemia and hyperglycemia increase myocardial lipid content in normal subjects. Diabetes 2012; 61:1210-1216.
- Borai A., Livingstone C., Ferns G.A. The biochemical assessment of insulin resistance. Ann Clin Biochem 2007; 44:324-342.
- Balsan G.A., Vieira J.L., Oliveira A.M., Portal V.L. Relationship between adiponectin, obesity and insulin resistance. Rev Assoc Med Bras 2015; 61:72-80.
- Freitas Lima L.C., Braga V.A., do Socorro de Franca Silva et al. Adipokines, diabetes and atherosclerosis: an inflammatory association. Front Physiol 2015; 6:304.
- Wallace T.M., Levy J.C., Matthews D.R. Use and abuse of HOMA modeling. Diabetes Care 2004; 27:1487-1495.
- Pacini G., Mari A. Methods for clinical assessment of insulin sensitivity and beta-cell function. Best Pract Res Clin Endocrinol Metab 2003; 17:305-322.
- Matsuda M., DeFronzo R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22:1462-1470.
- Stumvoll M., Mitrakou A., Pimenta W. et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000; 23:295-301.
- Welch S., Gebhart S.S., Bergman R.N., Phillips L.S. Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab 1990; 71:1508-1518.
- Levy-Marchal C., Arslanian S., Cutfield W. et al. Insulin resistance in children: consensus, perspective, and future directions. J Clin Endocrinol Metab 2010; 95:5189-5198.
- Pfutzner A., Derwahl M., Jacob S. et al. Limitations of the HOMA-B score for assessment of beta-cell functionality in interventional trials-results from the PIOglim study. Diabetes Technol Ther 2010; 12:599-604.
- DeFronzo R.A., Tobin J.D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237:E214-E223.
- Bloomgarden Z.T. Measures of insulin sensitivity. Clin Lab Med 2006; 26:611-33, vi.
- Wallace T.M., Matthews D.R. The assessment of insulin resistance in man. Diabet Med 2002; 19:527-534.
- Ferrannini E., Mari A. How to measure insulin sensitivity. J Hypertens 1998; 16:895-906.
- Visentin R., Dalla M.C., Basu R., Basu A., Rizza R.A., Cobelli C. Hepatic insulin sensitivity in healthy and prediabetic subjects: from a dual- to a single-tracer oral minimal model. Am J Physiol Endocrinol Metab 2015; 309:E161-E167.
- Altaf Q.A., Barnett A.H., Tahrani A.A. Novel therapeutics for type 2 diabetes: insulin resistance. Diabetes Obes Metab 2015; 17:319-334.
- Bailey C.J. Treating insulin resistance: future prospects. Diab Vasc Dis Res 2007; 4:20-31.
- Cohen P. The twentieth century struggle to decipher insulin signalling. Nat Rev Mol Cell Biol 2006; 7:867-873.
- Mahadev K., Zilbering A., Zhu L., Goldstein B.J. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem 2001; 276:21938-21942.




