Dipeptidyl peptidase-4 (DPP-4) inhibitors are promising anti-hyperglycemic agents, representing a major therapeutic advance for patients with type 2 diabetes as they do not induce hypoglycemia or weight gain and are generally well tolerated.
Sitagliptin is an orally administered DPP-4 inhibitor and first of its kind on the market (2006). It prolongs the action of incretin hormones glucagon like peptide-1 (GLP-1) and glucose-dependant insulinotropic polypeptide (GIP), by inhibiting their breakdown. Hereby it increases the secretion of insulin and supresses the release of glucagon, which helps to drive blood glucose values towards normal.
Why has the study been conducted?
Since 2008, regulatory agencies are requiring an assessment of cardiovascular disease (CVD) safety for the approval of all new anti-hyperglycaemic agents, including incretin-based therapies. To date, three large prospective DPP-4 inhibitor trials (including TECOS) have been published1-3.
TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin) was specifically designed to confirm that sitagliptin plus usual care did not increase the risk (non-inferiority with a marginal upper boundary of 1.3) for the primary composite cardiovascular outcome defined as the first confirmed event of cardiovascular death, nonfatal myocardial infarction (MI), nonfatal stroke, or hospitalisation for unstable angina compared to placebo plus usual care. Other secondary outcomes included the occurrence of the individual components of the primary composite outcome, fatal and nonfatal MI, fatal and nonfatal stroke, death from any cause, and hospitalization for heart failure.
Design of the study
TECOS was a large-scale cardiovascular-outcomes study that enrolled 14,671 patients from 38 countries with type 2 diabetes and established cardiovascular disease between December 2008 and July 2012. At baseline, the mean HbA1c level was 7.2% (with an HbA1c range of 6.5% to 8.0%) and patients had been living with their diabetes for 11.6 years. The compact range of baseline Hb1Ac was intended to minimize any effects of differences in glucose control on CV results. The median patient follow-up period was 2.8 years. The study was completed after having reached the minimum of 1300 patients who confirmed to have the primary outcome.
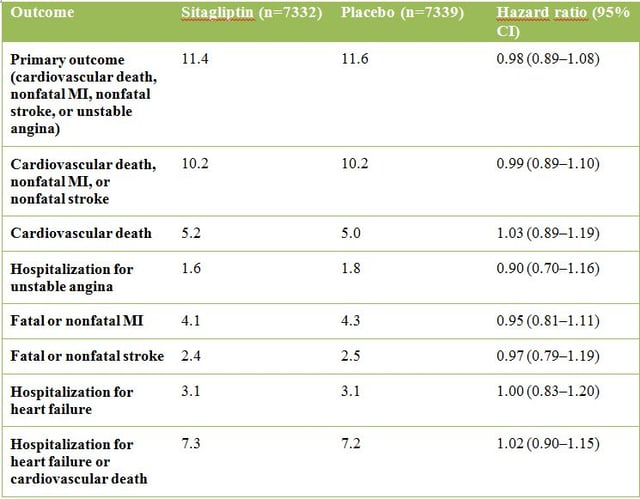
Results
During the median follow-up of 2.8 years, there was a slight difference in Hb1Ac levels (with a difference of 0.4% at the beginning, which later decreased to 0.1% as patients were treated to achieve their glycemic goals during follow-up). Overall, the difference was -0.29% in the sitagliptin-treated patients compared to placebo.
Regarding the primary end point there was no significant difference between sitagliptin and placebo treatment (see table below). By excluding unstable angina from the primary end point, there was also no significant increase in the risk of cardiovascular death, nonfatal MI, or nonfatal stroke. Altogether, there was no signal of cardiovascular risk among any of the end points when analysed separately or in combination.
Importantly, no increase in the number of patients hospitalized for heart failure was observed after sitagliptin treatment. This secondary end point is watched critically, since other drugs in the class, namely saxagliptin (Onglyza, AstraZeneca) in the SAVOR-TIMI 53 study, may have increased the risk of heart-failure events. Also the EXAMINE study, investigating type 2 diabetic patients treated with alogliptin (Nesina, Takeda Pharmaceuticals), showed a tendency towards an increased risk of heart-failure. However, further exploration is needed to assess these potential effects of the different drug classes.
Confirmed acute pancreatitis was uncommon, occuring in 0.3% of patients (n=23) in the sitagliptin group and 0.2% in the placebo group. Pancreatic cancer was also uncommon, appearing in 0.1% (n=9) and 0.2% (n=14) of the patients in the sitagliptin and placebo group, respectively.
Conclusion
TECOS CV safety results show that treatment with sitagliptin did not increase the risk of major adverse CV events in the primary composite endpoint and did not increase the risk of hospitalization for heart failure in a diverse group of type 2 diabetes patients.
The study was supported by Merck, Sharpe, & Dohme. Disclosures for the co-authors are listed on the journal website (http://www.nejm.org/doi/suppl/10.1056/NEJMoa1501352/suppl_file/nejmoa1501352_disclosures.pdf).
References
- Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR, Group TS. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2015; 373(3): 232-42.
- Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I, Committee S-TS, Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369(14): 1317-26.
- White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F, Investigators E. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369(14): 1327-35.




