No influence of calorie-free drinks on total daily energy intake compared to sugar-sweetened drinks, recent study says
Worldwide, the incidence of obesity is increasing dramatically having more than doubled since 1980 [1]. Because obesity is associated with an array of metabolic pathologies, including cardiovascular disease, type 2 diabetes, musculoskeletal disorders, and even some cancers, identifying strategies that help regulate body weight is imperative.
Substituting nutritive sweeteners by non-nutritive sweeteners (NNS), including artificial and natural NNS, may have potential in facilitating weight control [2-4]. By preserving palatability despite having fewer calories than sugar, NNS could help to lower the energy density of beverages and foods, resulting in a lower total energy intake. However, recurring arguments indicate that NNS increase the appetite for sweet foods, promote overeating, and may even lead to weight gain [5-7]. In light of that, numerous studies in the past two decades have been performed to address these issues, with the overall question remaining: Do NNS help to reduce body weight?
The question whether NNS have an effect on appetite and energy intake was recently addressed by Tey and colleagues [8] of the Agency of Science, Technology and Research in Singapore. Four different drinks were tested: one containing sugar (sucrose), one containing the artificial NNS aspartame, and two others – natural NNS – made from the plant stevia (rebaudioside A) or monk fruit (mogroside V). To date, this is the first study which investigated the effects of monk fruit- and stevia-containing preloads; with stevia approved in the EU in 2011, and monk fruit as novel NNS, which has not yet been approved in the EU but has been granted GRAS status in the US.

Design & Methods
Thirty healthy male subjects were enrolled in this randomised, double-blind, crossover study with four different treatments: aspartame-, monk fruit-, stevia-, and sucrose-sweetened beverages. On each of the four test days, subjects were provided with a standardised breakfast which they had to consume in the morning (between 08:00 and 09:00 hours) before the intake of the test beverage in the mid-morning at the study site (at around 11:00 hours). An ad libitum lunch was provided an hour thereafter, where participants were asked to eat until they were comfortably full. Blood glucose and insulin concentrations were measured closely (every 15 minutes in the first hour, and every 30 minutes for the subsequent two hours) until participants left the study site three hours after preload consumption. For the rest of the day, subjects had to complete a food diary as well as taking pictures of all foods they had consumed.
Results
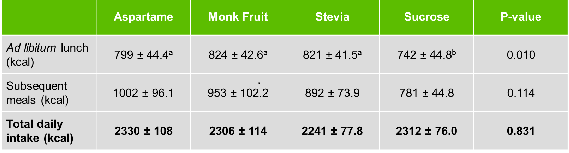
Tey et al. were able to show that ad libitum lunch intake was significantly higher for all three NNS treatments compared to sugar (see table 1). The energy ‘saved’ with NNS compared to sugar was fully compensated over the course of the day, meaning that no difference could be observed for total daily calorie intake between all four treatments. Participants either reduced meal intake after the sugar-sweetened drink or ate significantly more at lunchtime and the rest of the day to compensate for the three calorie-free drink options.
Table 1. Energy intake for lunch, subsequent meals, and total daily intake for each treatment (mean ± s.e.; n=30). Rows with different lower case numbers are significantly different from each other.
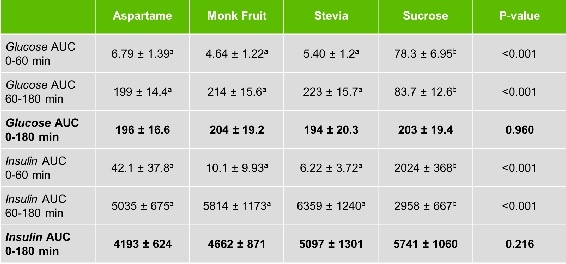
As expected, Tey et al. also demonstrated that the consumption of the sugar-containing beverage led to large glucose and insulin peaks within the first hour (with no increase for the NNS-containing beverages). In contrast, responses for all three NNS were higher following the ad libitum test lunch. Over the 3-hour observation period, there were no differences in total area under the curve for glucose and insulin between the four test drinks (see table 2).
Table 2. Glucose and insulin area under the curve (AUC) for each treatment (mean ± s.e.; n=30). Rows with different lower case numbers are significantly different from each other.
These results also indicate that the source of NNS, whether artificial or natural, does not differ in its effects on overall energy intake, postprandial glucose and insulin excursions.
Discussion
One of the concerns with the use of NNS is increased appetite, possibly leading to an overconsumption of food and a reversal of the energy-saving effect of NNS. In the present study overall caloric intake over the course of the day was comparable between NNS and sugar containing beverages, which is supported by a recent review describing no increase or preference for sweet taste as well as no increase in energy intake after NNS consumption [9].
The present study is contrary to previous findings suggesting that NNS consumption could reduce overall energy intake. However, it is important to note that this study examined a simple exchange of one serving of sugar-containing beverage and that the design of the trial does not allow a conclusion about long-term effects. In addition, the study investigated males with normal BMI only, which could not be carried forward to an obese population.
A former meta-analysis including 15 randomized controlled trials showed that NNS consumption over 4-26 weeks led to a modest weight loss (-0.80 kg reduction in body weight compared to the competitor arm). None of these studies showed weight gain with NNS consumption [10].Another recent meta-analysis including 12 sustained intervention studies reached similar conclusions [11]. In this meta-analysis, a body weight loss of -1.35 kg versus sugar and -1.24 kg versus water was observed. In line with the present study, no increase in energy intake with the use of NNS in human studies is reported in the literature.
In light of postprandial glycemia as risk factor for diabetes and cardiovascular disease, Tey and colleagues discussed the large spikes after the ad libitum lunch, which were greater after intake of the three NNS than after the sugar-containing preload. The authors argued that consuming NNS alone without any energy (empty calories) before a meal may lead to larger spikes in glucose and insulin responses after the meal. In contrast, when subjects consume NNS together with caloric foods as a preload, postprandial glucose concentration following lunch was significantly lower with NNS compared to the sugar-sweetened preload [12]. A previous study was able to show that a diet rich in sugar (2 g per kg body weight) for 10 weeks led to significantly higher postprandial glucose concentrations compared to a diet rich in NNS [13]. It might be possible that a higher number of exposures or a longer intervention period is necessary in order to observe beneficial effects of NNS on glycemia and insulinemia.
Conclusion
The consumption of calorie-free preloads sweetened with NNS (artificial and natural) does neither affect total daily energy intake, nor glucose and insulin responses compared with a sugar-containing preload in healthy lean males in this short term setting.
References
- World Health Organization, Obesity and overweight Fact sheet N°311 January 2015.
- Fitch C, Keim KS, Academy of N, Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 2012; 112(5): 739-58.
- Anderson GH, Foreyt J, Sigman-Grant M, Allison DB. The use of low-calorie sweeteners by adults: impact on weight management. J Nutr 2012; 142(6): 1163S-9S.
- Drewnowski A. Intense sweeteners and energy density of foods: implications for weight control. Eur J Clin Nutr 1999; 53(10): 757-63.
- Erlanson-Albertsson C. How palatable food disrupts appetite regulation. Basic Clin Pharmacol Toxicol 2005; 97(2): 61-73.
- Ninomiya Y, Shigemura N, Yasumatsu K, Ohta R, Sugimoto K, Nakashima K, Lindemann B. Leptin and sweet taste. Vitam Horm 2002; 64: 221-48.
- Schwartz MW. Central nervous system regulation of food intake. Obesity (Silver Spring) 2006; 14 Suppl 1: 1S-8S.
-
Tey SL, Salleh NB, Henry CJ, Forde CG. Effects of non-nutritive (artificial vs natural) sweeteners on 24-h glucose profiles. Eur J Clin Nutr 2017.
-
Bellisle F. Intense Sweeteners, Appetite for the Sweet Taste, and Relationship to Weight Management. Curr Obes Rep 2015; 4(1): 106-10.
-
Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr 2014; 100(3): 765-77.
-
Rogers PJ, Hogenkamp PS, de Graaf C, Higgs S, Lluch A, Ness AR, Penfold C, Perry R, Putz P, Yeomans MR, Mela DJ. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int J Obes (Lond) 2016; 40(3): 381-94.
-
Anton SD, Martin CK, Han H, Coulon S, Cefalu WT, Geiselman P, Williamson DA. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite 2010; 55(1): 37-43.
-
Raben A, Moller BK, Flint A, Vasilaris TH, Christina Moller A, Juul Holst J, Astrup A. Increased postprandial glycaemia, insulinemia, and lipidemia after 10 weeks' sucrose-rich diet compared to an artificially sweetened diet: a randomised controlled trial. Food Nutr Res 2011; 55.
Stay up to date on this and other issues. Sign up for our monthly blog post update (on the right of this page). Else, learn more about implantable CGMs and watch our video on the topic; click here.




