Cardiovascular (CV) events are the main cause of death among patients with type 2 diabetes mellitus (T2DM). Myocardial infarction (MI) and stroke are the macrovascular complications responsible for the high mortality in this collective of patients (1). The molecular mechanisms occurring in insulin resistant subjects contribute directly to the pathogenesis of atherosclerosis, independently of the concomitant metabolic abnormalities (2). Improvements in HbA1c alone take years if not decades to impact the CV risk profile (3), meaning that glycemic control alone is of poor utility to demonstrate reduced mortality of diabetes patients in short-term clinical trials. For all these reasons, developing glucose-lowering drugs that tackle the high CV risk is of interest for the medical community.
The approval of new therapies for any disease includes the accumulation of results showing efficacy and safety of the drug in development. From the safety point of view it is important that the new substances demonstrate that there is an acceptable risk in taking the drug. Given the importance of the CV events in the diabetic population it is a regulatory requirement to prove that the occurrence of these events is not worse than in the population taking placebo. The U.S. Food and Drug Administration (FDA) released a guidance for the industry (4) given the concerns of certain glucose-lowering drugs impacting negatively CV mortality. This guidance defines, as a regulatory standard, the execution of trials using CV outcomes for evaluating the risk of antidiabetic therapies (4).
The initial aim of such studies is to demonstrate non-inferiority compared to placebo regarding a number of CV outcomes. Since the low occurrence of these events does not permit the investigation of each outcome separately, it has become common practice to use composite primary endpoints in trials investigating glucose-lowering medications. Composite endpoints consistent of 3-point major adverse cardiac events (MACE) typically include: CV death, non-fatal MI, and non-fatal stroke. The 4-point MACE includes additionally hospitalisation for unstable angina (HUA) and 5-point MACE adds hospitalisation for heart failure. In Table 1 a selection of trials using composite endpoints is presented.
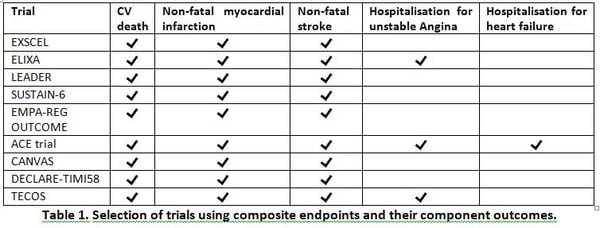
The selection of the component outcomes seems to have a big influence on the results. In fact, among the over 25 trials that have been performed in last 15 years only 4 have shown superiority in terms of reduction of CV outcomes: EMPAREG-OUTCOME (5), SUSTAIN-6 (6), CANVAS (7) and LEADER (8). These four trials had in common the use of the same 3-point MACE in a single composite outcome (Table 1). Whether the selection of endpoints, other design aspects or the drug itself had a determining influence on the results is still a matter of discussion. This observation follows from the fact that similar designs with medications of the same class showed dissimilar results. The case of the EXSCEL trial is interesting, since it showed improvements in all-cause mortality, while the improvements in the composite CV endpoint just missed statistical significance (9).
Using composite endpoints is still a matter of intense debate. There is no consensus regarding which component outcomes to use and whether the composite endpoints are overall valid for the evaluation of CV risk in diabetes trials.
Advantages and disadvantages of composite endpoints
The main advantage of having a composite primary endpoint is the increase in power. The basic principle supporting its use is that the chances of reaching a composite endpoint are higher than the single outcomes taken alone. Using a single component might lead to the necessity of investigating extremely large samples in order to detect sufficient events for adequate statistical power.
The risk of including composite endpoints is that when the effects of the components are heterogeneous the overall effect of the composite is diluted. This can lead, in some cases to a high probability of false negative interpretations. For instance, a GLP-1 receptor agonist might decrease the risk of stroke or myocardial infarction, but might not have a large effect on hospitalization for heart failure. The opposite would happen in studies with SGLT-2 inhibitors, which have a strong diuretic effect, and therefore, an improvement in the rates of hospitalisation due to heart failure could be expected.
Hospitalisation for unstable angina as outcome
Using HUA is frequently problematic because clear criteria for both the diagnosis unstable angina and the decision for hospitalization are lacking. Frequently there is discordance between the assessment of local physicians and trial investigators. Overall, the hazard ratio of HUA in studies with glucose-lowering medications ranges from 0.82 to 1.19, meaning that adding HUA to the composite outcome might well lead to a demonstration of non-inferiority. Therefore, despite sharing the same pathophysiological mechanism with myocardial infarction, using it as component endpoint might dilute, in some cases, the effect of the treatment (10).
Using hospitalisation for heart failure as outcome
Using hospitalisation for heart failure in composite outcomes has been frequently discussed (11). This is important because hospital admission for heart failure is a common cardiovascular complication of patients with diabetes. It is usually defined as secondary endpoint, although in light of the mechanisms of action of SGLT2 inhibitors it would be of interest for the diabetes community to know the precise effect of the glucose-lowering medications on this outcome.
Conclusion
In conclusion, Composite endpoints are useful for the evaluation of the effect of glucose-lowering medications on CV risk, but there is no mutual agreement on one pre-defined composite endpoint applicable for all trials. It rather seems that the choice of outcomes should be made in light of the mechanism of action and the expected benefit of the drugs.
References
- Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. The New England journal of medicine. 2011;364(9):829-41.
- DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53(7):1270-87.
- Gerstein HC, Miller ME, Byington RP, Goff DC, Jr., Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-59.
- U.S. Food and Drug Administration. Guidance for industry: diabetes mellitus valuating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes [Available from: https://www.fda.gov/downloads/Drugs/Guidances/ucm071627.pdf.
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117-28.
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. The New England journal of medicine. 2016;375(19):1834-44.
- Fernandez-Balsells MM, Sojo-Vega L, Ricart-Engel W. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. The New England journal of medicine. 2017;377(21):2098.
- Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. The New England journal of medicine. 2016;375(4):311-22.
- Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. The New England journal of medicine. 2017;377(13):1228-39.
- Marx N, McGuire DK, Perkovic V, Woerle HJ, Broedl UC, von Eynatten M, et al. Composite Primary End Points in Cardiovascular Outcomes Trials Involving Type 2 Diabetes Patients: Should Unstable Angina Be Included in the Primary End Point? Diabetes care. 2017;40(9):1144-51.
- McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. The lancet Diabetes & endocrinology. 2014;2(10):843-51.




