Part I: Introdution and Basic UDI-DI
Introduction
In this first of two blog posts, we will describe the unique device identifier (UDI) system required for medical devices according to the European Medical Device Regulation (MDR). Part I of this series will indroduce the Basic UDI-DI, whereas Part II is about the unique device identifier (UDI-DI) and the production identifier (UDI-PI).
Regulation (EU) 2017/745 of the European Parliament and of the Council (MDR) [1] introduces the UDI system for unique product identification of medical devices. The UDI system will facilitate easier traceability of medical devices, significantly enhance the effectiveness of the post-market safety-related activities for devices and allow for better monitoring by competent authorities. It will also help to reduce medical errors and combat falsified devices.
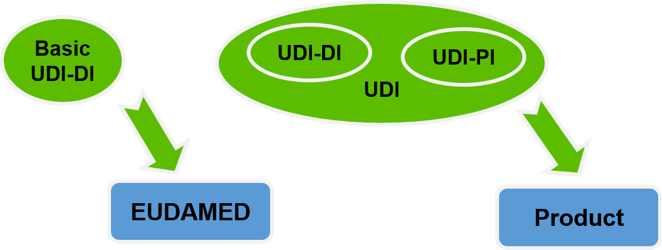
The UDI essentially consists of the Basic UDI-DI, the Device Identifier (UDI-DI), the Production Identifier (UDI-PI), and the registration of the manufacturer and the medical product in the Eudamed database (Eudamed) [2].
-
The Basic UDI-DI is a higher-level key for product groups from a manufacturer with common properties. It is the leading key for product-related vigilance information in Eudamed and is not placed on the product or packaging.
-
The Device identifier UDI-DI is an alphanumeric product key that is assigned to the medical device. Different packaging levels with different pack quantities need their own UDI-DI, whereas shipping containers are exempt.
-
The Production identifier UDI-PI is a manufacturing feature that identifies the production pa-rameters (batch/lot/production date/expiry date) of the product. The combination of UDI-DI and UDI-PI is called UDI.
-
Eudamed is the European database for medical devices managed by the European Com-mission.
To create a Basic UDI-DI and UDI the involvement of a UDI issuing entity is needed. All issuing enti-tites operate a system for the assignments of UDIs. The following three issuing entities are included in the MDR:
- GS1 AISBL
- Health Industry Business Communications Council (HIBCC)
- International Council for Commonality in Blood Banking Automation (ICCBBA)
Additionally, by means of an implementing decision of the European Commission a forth issuing entity was appointed: - Informationsstelle für Arzneispezialitäten (IFA) GmbH [3]
Each manufacturer is free to choose one of the issuing entities according to their preferences and/or history. The role of each of the issuing entities is to assign a unique company identification number to each of the applying manufacturers.
As an example for Profil as the manufacturer of the ClampArt device, Profil selected IFA as issuing entity. Therefore, the data elements required to implement the UDI specifications from the MDR were generated via the IFA Coding System. The IFA Coding System is al-ready being used successfully in the pharmaceuticals sector to implement the EU directive relating to medicinal products for human use, as regards the prevention of the entry into the legal supply chain of falsified medicinal products.
The unique Company Identification Number (CIN) for Profil as a manufacturer for medical devices assigned by IFA is: 12091
With this unique Company Identification number the Basic UDI-DI and UDI can be created.
Basic UDI-DI
- Certificates issued by a notified body,

- Declaration of conformity,
- Technical Documentation,
- Summary of Safety and Clinical Performance,
- Vigilance reports,
- Periodic Security Update Report (PSUR).
The Basic UDI is assigned before the medical device is placed on the market.
With the Basic UDI-DI, the manufacturer forms groups of products with common properties. The Basic UDI-DI does not appear on the packaging or on the product.
The Basic UDI-DI is generated from
-
An identifier of the named issuing agency code (IAC),
-
The company identification number assigned by the named issuing agency (CIN),
-
The device group code (DGC) and
-
A two-digit control code.
To determine the control number, the individual characters in the character string (IAC+CIN+DGC) are converted into their ASCII code. Each digit of this string is weighted with a factor starting with 2 and increasing by 1 from left to right. The weighted digits are then added up. The control number is the remainder after dividing this weighted sum by 97.
For example, the Basic UDI-DI for the medical device “ClampArt“ with the manufacturer Profil is created as follows:
The Issuing Agency Code for IFA as the issuing entity ist “PP“.
The Company Identification Number for Profil, asssigned by IFA ist “12091“
Profil selected “ClampArt“ as the device group code for the ClampArt device.
The resulting character string is “PP12091CLAMPART“.
To generate the control number, each of the characters is converted to its ASCII code (e.g. 80 for character ‚P‘, 49 for character ‚1‘, …) and then assigned a weighting factor starting with 2.
2*80(P) + 3*80(P) + 4*49(1) + 5*50(2) + 6*48(0) + 7*57(9) + 8*49(1) + 9 *67 (C) + 10*76 (L) + …

The manufacturer has to ensure that a device group code is not used more than once for different products to keep this Basic UDI-DI unique across Europe. To ensure this the manufacturer has to keep a UDI allocation list with all assigned device group codes and doublecheck new device group codes against all entries in this list.
The device identifier (UDI-DI) and the production identifier (UDI-PI) will be addressed in the next part of this blog series.
Read more about our expertise in testing medical technology: Insulin pump development and design, artificial pancreas study and the AP@home project, glucose monitoring.




