Dual- and multi-receptor agonists may be a novel drug class to treat obesity and type 2 diabetes mellitus with high efficacy
The rising prevalence of obesity has metabolic consequences such as diabetes and cardiovascular complications. Many existing therapies for type 2 diabetes mellitus (T2DM) focus on lowering blood glucose; however, there is a major unmet need for treatments that both improve glycemic control and achieve metabolic benefits by weight loss. Lifestyle interventions, such as dieting and physical activity, typically provide only short-lasting weight loss in obese people, as weight loss maintenance is the greatest barrier to successful treatment of obesity [1].
Targeting the incretin system [e.g. with Glucagon-like peptide (GLP-1)]
A pharmacological approach targeting the incretin system, e.g. with GLP-1, may be considered for weight reduction. Native GLP-1 is a peptide hormone produced within the L-cells of the intestine and is secreted in response to nutrient ingestion, especially in response to meals high in fat and carbohydrates [2]. GLP-1 delays gastric emptying, stimulates insulin secretion in pancreatic beta-cells, and mediates satiety in the brain, actions that are beneficial to individuals with obesity and T2DM. Nausea and vomiting are the most common dose dependent side effects which can limit the use of higher doses to drive greater weight loss [3].
Bariatric surgery
Weight reduction and glycemic control achieved with certain types of gastric bypass surgery show markedly superior outcomes compared to pharmacological treatments [4, 5, 6]. As the intervention is highly invasive, has considerable risks and cannot be reversed, surgery is not a viable option for most patients and mainly indicated in people who have a BMI of more than 40 kg/m2 or of more than 35 kg/m2 and at least one obesity-related comorbidity [7]. Improvement in glucose control after gastric bypass or sleeve gastrectomy surgery occurs rapidly, and may involve mechanisms such as an enhanced incretin effect, and gut-brain neuronal pathways, which could be responsible for improved glucose control, independent of weight loss [8].
Targeting the incretin/glucagon system: the effects of native oxyntomodulin
Native oxyntomodulin (OXM) is a naturally occurring incretin, co-secreted from the intestinal L-cells with GLP-1 in response to nutrient intake. OXM can activate both GLP-1 and glucagon (GCG) receptors. Dose finding studies in rodents using OXM analogues have shown that there is a non-linear balance between the anorectic effect of GLP-1 receptor activation and GCG receptor-mediated energy expenditure [9, 10].
Glucagon (GCG) the main catabolic hormone of the body
GCG is a peptide hormone secreted by the alpha-cells of the pancreas in response to fasting or hypoglycemia [11]. Its primary physiological role is to raise blood glucose levels by inhibiting insulin secretion and by stimulating hepatic glucose production. When insulin resistance occurs (as in obesity and T2DM) or when there is complete absence of endogenous insulin (as in T1DM), there is relative hyperglucagonaemia. Owing to glucagon’s hyperglycemic and insulin-suppressing effects, the glucagon receptor has historically been a prime target for pharmacological suppression rather than activation in a therapy for obesity and, certainly, diabetes [12, 13, 14]. However, GCG is considered to be the main catabolic hormone of the body and has similar effects to GLP-1 on gastric emptying and appetite, and has also been shown to promote energy expenditure [15, 16].
Targeting the incretin/glucagon system with dual-agonists
Improved metabolic efficacy could therefore be achieved by combining multiple metabolic actions by pairing the well-established pharmacology of GLP-1 to GCG, a combination which at first glance does not appeal intuitively. Native OXM invokes a dual component pathway to glucose lowering, a direct weight-loss independent component that has an acute onset, and an indirect component via weight loss that has a more gradual onset and protracted time course profile which has been shown in obese humans with and without diabetes [17]. Thus, the new compounds targeting a balanced GLP-1 and GCG activation are based partly on the effects of the natural gut hormone oxyntomodulin [17].
Metabolic actions of GLP- 1/ GCG receptor co-agonists on key organs [see Figure 1]
Recently developed unimolecular GLP-1/GCG receptor co-agonists have elicited robust glycemic control and promote sustained weight loss in animals [18, 19, 20] and have shown superior preclinical efficacy compared to currently prescribed monotherapies in the treatment of obesity. The action of dual GLP-1/GCG receptor agonists probably results from a combination of central and peripheral mechanisms at multiple target tissues to regulate energy and glucose homeostasis [21]. Dual GLP-1/GCG receptor agonists suppress food intake and increase satiety and leptin sensitivity (an adipocyte-derived hormone known to regulate energy homeostasis to control energy and glucose metabolism) by acting in the brain [22]. In addition to effects on glucose homeostasis, glucagon has both catabolic and thermogenic actions by increasing brown adipose tissue (BAT) thermogenesis [23, 24]. However, recent evidence that glucagon increases energy expenditure independently of BAT activation in humans [25] indicates that alternative mechanisms such as futile substrate cycling [26] may underlie glucagon’s thermogenic properties. The observations regarding a mechanism-related switch of substrate oxidation can be of further therapeutic benefit, and might explain the superior effect of dual GLP-1/GCG receptor agonists for body weight and glucose control, which has been observed in animals [27, 28] and in obese and diabetic patients [29]. Glucagon administration also decreases hepatic triacylglycerol synthesis in rats [30] and stimulates hormone-sensitive lipase in human and rat white adipocytes to promote lipolysis and the release of non-esterified fatty acid (NEFA) [31, 32]. These fatty acids freely circulate and access heart, skeletal muscle, kidneys and liver [29]. The kidneys and liver metabolise the fatty acids, producing ketone bodies as common metabolites [29]. Long-term intervention with the dual-action peptide improved lipid metabolism and hepatic steatosis when compared with a chemically matched peptide which has high selectivity for the GLP-1 receptor [33]. Contrary to expectations, glycemic control was also improved after chronic treatment with the GLP-1/GCG receptor co-agonist in rats, which implies that GLP-1 activity protected against glucagon-induced hyperglycemia [19]. Despite its low potency at both receptors and less-certain in vivo activity at the GCG receptor, chronic dual agonist treatment decreased body weight and food intake in rodents when GLP-1 receptor action was present [34, 35, 36] making a further development of balanced dual agonist for treatment of diabetes and obesity a promising approach [37].
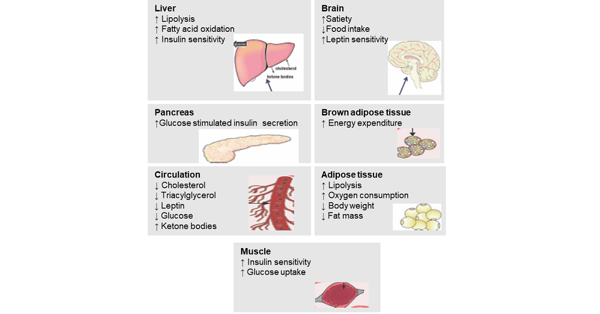
Figure 1: Metabolic actions of GLP- 1 and GCG receptor agonists on key organs [brain, (brown) adipose tissue, muscle, liver, pancreas and circulation] regulating energy and glucose homeostasis and changes in metabolic variables (modified according to Sánchez-Garrido MA et al. GLP-1/glucagon receptor co-agonism for treatment of obesity. Diabetologia. 2017.).
Synthetic oxyntomodulin-like peptide in clinical development
Synthetic oxyntomodulin-like peptides with targeted glucagon-like peptide 1 (GLP-1) and glucagon receptor activity are under development for the treatment of T2DM, obesity, and non-alcoholic steatohepatitis (NASH). First clinical results have shown positive metabolic treatment effects on glycemic control, body-weight, and liver fat in overweight and obese people with T2DM over a relatively short period of dosing. The safety and tolerability results promoted continued development and suggest that with longer-term therapy, synthetic balanced dual-agonists have the potential to be a disease-modifying therapy for T2D [38]. While the long-term consequences of GLP-1 receptor agonism are now emerging, similar long-term studies will be required to investigate the safety of new co-agonists in the brain and cardiovascular system, and their potential for promoting cell proliferation [39]. So far, the phase II trial results of the GLP-1/GCG dual agonists support commencement of a phase III pivotal program.
Targeting the incretin/glucagon system with multi-receptor agonists
Furthermore, novel GLP-1/GCG hybrid peptides with triple-acting agonist activity at glucose-dependent insulinotropic polypeptide (GIP), and GLP-1/GCG receptors have been developed which amplify the well-established physiological incretin effect and appear to have powerful insulin-releasing, glucoregulatory properties and pancreatic beta-cell protective actions [40].
The development of balanced, unimolecular dual- and multi-receptor agonists may provide a novel drug class to treat obesity and T2D with high efficacy in the near future [41].




