The glucose clamp methodology can be used in different configurations to investigate a variety of research questions and is regarded as gold standard for insulin sensitivity assessments and time-action profiling of glucose-lowering drugs. At Profil, we are always thinking of innovative ways to answer specific research questions with this unique methodology. The combination of glucose clamp methodology and blood glucose systems (meters and test strips) may not seem obvious, but can actually provide special opportunities for performance evaluation testing of glucose measurements as Profil scientists show in their recent publication in the Journal of Diabetes, Science and Technology [1].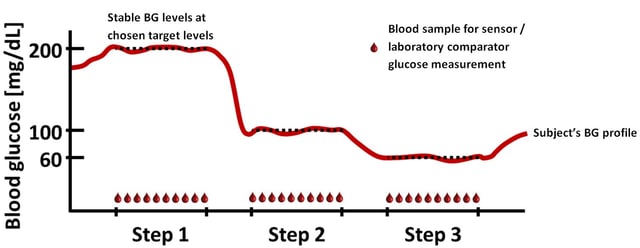
Clamp technology was used in the study to keep the blood glucose of a subject stable at a target level for a longer period of time. During the time with stable blood glucose, frequent blood samples were taken and measured in parallel by blood glucose systems and a laboratory glucose analyser. Three target levels were used in the study to obtain paired data at low, normal and high glucose levels. This approach provides a unique opportunity to assess the random error or measurement variability of a glucose measuring device in vivo over a clinically significant range. This would not be possible in a human being without the clamp technology, because the blood glucose would vary too much on its own to allow the measurement variability assessment. Current performance evaluations of blood glucose systems therefore have to revert to laboratory tests for assessing measurement variability, which is not ideal as samples are spiked with glucose or diluted to achieve high and low glucose concentrations and maintaining a good quality blood sample in the lab (for example with regard to its oxygenation) can be challenging.
The development of this method therefore provides a different level of evaluation to test the measurement quality of blood glucose systems, a topic that has been fiercely debated at conferences and workshops over the last years since reports of poorly performing CE-certified and FDA-cleared systems have come out [2, 3] (click to read our blog post on this topic).
The study evaluated the performance of five blood glucose systems, which require a capillary blood sample for a measurement. However, the advantage of the published method becomes even greater for glucose measurement devices that do not test in blood, such as CGM systems with interstitial glucose measurements or non-invasive glucose monitoring devices. For these systems, a blood laboratory test is not an option and alternative variability assessments do not exist. Especially for CGM systems, the combination with clamp methodology may provide further unique opportunities: the first ‘rate clamps’, these are clamps in which not a stable glucose level is achieved, but a stable rate or change of glucose, have already been performed and will be presented later this year at the Diabetes Technology Meeting. In the near future, rate clamps may provide a standardised evaluation of measurement performance under dynamic conditions and a standardised assessment of measurement delay (time lag).
- Zijlstra E, Heinemann L, Fischer A, Kapitza C. A Comprehensive Performance Evaluation of Five Blood Glucose Systems in the Hypo-, Eu-, and Hyperglycemic Range. J Diabetes Sci Technol. 2016 Sep 6. [Epub ahead of print]
- Freckmann G, Schmid C, Baumstark A, Pleus S, Link M, Haug C. System accuracy evaluation of 43 blood glucose monitoring systems for self-monitoring of blood glucose according to DIN EN ISO 15197. J Diabetes Sci Technol. 2012;6(5):1060-75
- Heinemann L, Zijlstra E, Pleus S, Freckmann G. Performance of Blood Glucose Meters in the Low-Glucose Range: Current Evaluations Indicate That it is not Sufficient From a Clinical Point of View. Diabetes Care. 2015 Sep;38(9):e139-40.




