Once-weekly basal insulin icodec treatment may offer more convenience in people with type 2
diabetes and may improve outcomes.
Molecular and biological properties of once-weekly insulin icodec and promising phase 1 and 2 data
were presented at American Diabetes Association’s 80th Scientific Sessions and 56th European
Association for the Study of Diabetes (EASD) Annual Meeting 2020.
Insulin icodec is an acylated insulin analogue with three amino acid substitutions, leading to increased
stability and reduced proteolytic degradation. The acylation with a C20-icosane fatty diacid results in
a strong, but reversible binding of insulin icodec to albumin. After 3-4 weekly injections, steady state
is achieved, giving the full effect of insulin icodec dose with a slow and continuous release of insulin
icodec from the albumin-bound depot to the insulin receptor. Once bound to the insulin receptor,
insulin icodec elicits all metabolic effects as human insulin. The injection volume of once-weekly insulin
icodec is equivalent to a daily injection of basal insulin due to the concentrated formulation. At steady state,
variation in dosing time and amount are expected to lead to minimal changes in immediate
glucose-lowering effect [1].
Results from a 5-week multiple ascending dose trial performed at our institution in insulin treated
adults with type 2 diabetes supports a safe and well tolerated once-weekly administration at clinically
relevant dose levels. The long-acting basal insulin analogue exposure at steady-state showed
characteristics which are suited for once-weekly administration, in particular a half-life of
approximately one week at clinically relevant doses. Maximum concentration of insulin icodec was
observed within the first day after injection and clear dose-proportionality with higher doses leading
to higher exposure was demonstrated.
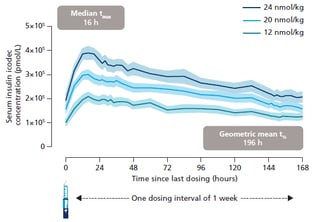
Figure 1: Mean serum insulin icodec concentration after multiple once-weekly
dosing in individuals with type 2 diabetes.
The graphs show total serum insulin icodec concentration (the vast majority being albumin-bound).
Error bands show standard error of the mean. AUC: area under the curve;
t½: half-life; tmax: time to maximum concentration
Furthermore, insulin icodec showed a close to even
distribution of the glucose lowering effect within a dosing interval of one week at steady-state and was
well tolerated [2,3].
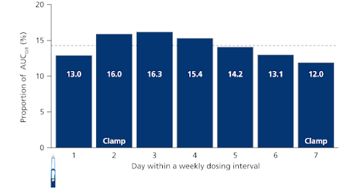
Figure 2: Modelled distribution of total glucose-lowering effect (AUC) of insulin icodec
within a dosing interval of one week at steady state
Dotted line represents equal distribution across 7 days. All 3 dose levels are combined.
Data are arithmetic mean; AUC: area under the curve; GIR: glucose infusion rate
The following phase 2 data, comprising three clinical trials, demonstrated the potential to offer a
simplified treatment option and compared different titration regimens with once-weekly insulin icodec
for people with type 2 diabetes initiating insulin treatment, as well as investigated two different
approaches to switch to once-weekly injection from other basal insulins.
Results from a 26-week phase 2 clinical trial in insulin-naïve adults with type 2 diabetes demonstrated
comparable blood sugar lowering and a similar safety profile of once-weekly insulin icodec compared
to once-daily insulin glargine U100 [4, 5, 6].
Another 16-week trial in insulin-naïve people with type 2 diabetes who were inadequately controlled
with oral antihyperglycemic drugs compared the effect of different titration algorithms of once-weekly
insulin icodec with once-daily insulin glargine U100 to better understand the optimal titration for a
once-weekly basal insulin. Insulin icodec was initiated at 70 units weekly and insulin glargine U100 at
10 units daily and titrated weekly based on self-measured blood glucose values. All three once-weekly
titration algorithms for insulin icodec investigated were shown to be well-tolerated and efficacious,
and demonstrated an improved or similar ‘time in range’ versus once-daily insulin glargine U100,
depending on the titration algorithm applied [7].
Results from a 16-week phase 2 clinical trial with adults with type 2 diabetes who were inadequately
controlled on oral antihyperglycemic drugs and once/twice-daily basal insulin demonstrated an easy
transition to once-weekly insulin. A unit to unit switch (or a 20% reduction for those receiving twicedaily
basal insulin or insulin glargine U300 prior to randomisation) with and without a 100 % loading
dose of insulin icodec was investigated. Data showed that switching to once-weekly insulin icodec from
other basal insulins using two different switch approaches was efficacious and well-tolerated
compared to once-daily insulin glargine U100 and demonstrated an improved or similar ‘time in range’
versus once-daily insulin glargine U100. There was no increased risk of clinically significant or severe
hypoglycemic episodes compared to once-daily insulin glargine U100 during the switching
approaches [8].
The findings in the phase 2 programme support the progression into phase 3 development and will
further guide the glycemic targets, titration algorithm, and switch approach in the succeeding phase
3 trials which are expected to start by the end of 2020.




