Non-invasive Continuous Glucose Monitoring (niCGM) is the holy grail of glucose monitoring. But the quest has been arduous so far; research carried out over the past 35 years has not resulted in in a device with durable availability on the market [1, accessed March 23, 2020].
Two devices have briefly been commercially available. The first, called GlucoWatch Biographer, has been for sale from 2002 to 2007. Poor local tolerability and low accuracy were the limiting factors. The device applied a low voltage current to obtain interstitial fluid transcutaneously. Skin irritation, probably inherent to this technique, together with insufficient accuracy, resulted in withdrawal from the market and dissolvement of the manufacturer [2].
The second device that reached the market obtained a CE mark around 2007, although it was probably sold to very few patients. Independent accuracy evaluation showed a Mean Absolute Relative Difference (MARD) of 52%, casting doubt on the key question whether glucose was measured at all [3]. MARD is the most widely accepted single number parameter reflecting CGM accuracy, taking the absolute value of each deviation of a CGM measurement to its paired reference glucose measurement, and then averaging these deviations. For comparison, the MARD of the first semi-invasive CGM devices entering the market around the turn of the century was around 20-25%, and the accuracy of current state-of-the-art semi-invasive devices is around 10%.
However, there are some clues indicating that the until recently so dire outlook for niCGM is changing. As can be seen from the graph, the number of new niCGM prototypes reported in the last few years seems to be on the rise, although some of these papers report results too good to be true, e.g. an MARD lower than any device on the market, without notable progress over a number of years. Also at Profil, we recently reported on a promising niCGM [4; Diabetes Technology & Therapeutics 2020;22(S1):A62].
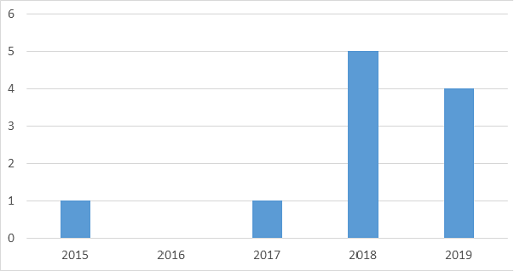
Figure. Number of studies reported in the medical literature according to a PubMed search on ‘non invasive continuous glucose monitoring’ and then selecting studies reporting on a prototype applied to humans
Nemaura Medical, a UK based company, recently entered the UK market with a device called sugarBEAT (https://sugarbeat.com/). A CE mark was obtained in 2019 and it is approved for adjunctive use. The device is submitted to FDA under the wellness category. Publications on its accuracy will hopefully follow soon. However, the need for calibration and the need for daily replacement seem to limit the differentiation versus flash glucose monitoring, although there may be differentiation in price.
Companies with health watches on the market that now already measure heart rate, saturation, sleep patterns and so on would of course much like to incorporate a niCGM into their device. Interest in personal glucose patterns is likely high, not only in people with diabetes, but also in healthy people. As some of these watches are brought to the market by companies with substantial resources, willingness to invest large amounts of money in the development of a niCGM may be expanding. So let’s hope that niCGM will evolve from holy grail into product on the market anytime soon!
 Figure. Mock-up prototype of a health watch non-invasive glucose monitor [5]
Figure. Mock-up prototype of a health watch non-invasive glucose monitor [5]




