Are GLP1 receptor agonists a treatment option in patients with non-alcoholic fatty liver disease (NAFLD)?
Nowadays many potential treatment options for diabetes mellitus are being evaluated for other diseases as well. For instance, GLP1-receptor agonists are found to have a positive effect on non-alcoholic fatty liver disease. NAFLD prevalence is increasing worldwide and affects up to 25% of the population [1]. Therefore at Profil a randomised, double-blind, placebo-controlled trial enrolled subjects with liver stiffness 2.50–4.63 kPa by magnetic resonance elastography (MRE) and liver steatosis ≥10% by MRI proton density fat fraction (MRI-PDFF). Primary endpoint was change from baseline to week 48 in liver stiffness assessed by MRE. Secondary endpoints included change from baseline to week 24, week 48 and week 72 on liver steatosis assessed by magnetic resonance imaging proton density fat fraction (MRI-PDFF).

Results
Of 266 subjects screened 67 subjects were randomised to subcutaneous semaglutide 0.4 mg (n=34) once daily or placebo (n=33). Of these, 27 subjects (79,4%) received semaglutide and 30 subjects (90,3%) placebo and completed the trial; hence, MRE-data for the primary endpoint were derived from 57 subjects at week 48. Baseline demographics and characteristics were well balanced between both groups.
Our evaluation for the change from baseline in liver stiffness showed no significant difference between semaglutide and placebo neither at week 48 (estimated treatment ratio 0.96 (95% CI 0.89, 1.03; P=0.2798) nor at weeks 24 or 72.
However, reductions in liver steatosis were significantly greater with semaglutide (estimated treatment ratios: 0.70 [0.59, 0.84], P=0.0002; 0.47 [0.36, 0.60], P<0.0001; and 0.50 [0.39, 0.66], P<0.0001) and more subjects achieved a ≥30% reduction in liver fat content with semaglutide (all P<0.001) at weeks 24, 48, and 72, respectively. [2]
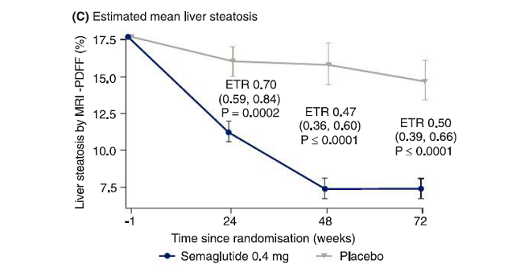
Fig. 1: ETR: Estimated treatment ratio
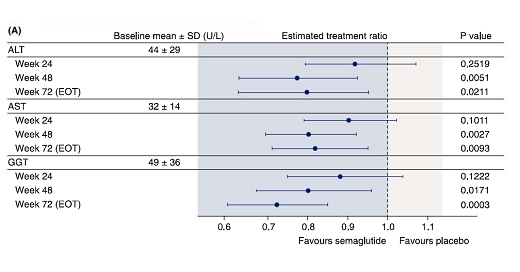
Moreover, decreases in liver enzymes, body weight, and HbA1c were observed with semaglutide.
Conclusion
Although the change in liver stiffness in subjects with NAFLD was not significantly different between semaglutide and placebo, the study results show a significant reduction for liver steatosis compared with placebo. Furthermore, metabolic parameters and liver enzymes improved with the active treatment of semaglutide. Currently, only lifestyle modification aiming for weight loss is the recommended therapy for NAFLD, i.e. no drug treatment is approved for NAFLD. Nevertheless, GLP-1 agonists may be part of the future therapeutic options for this disease.
If you are planning a similar trial, feel free to contact us. We are happy to discuss your next trial with you.




