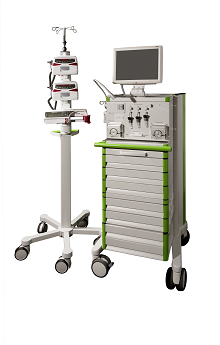
The euglycemic, hyperinsulinemic glucose clamp is the gold standard for the determination of pharmacokinetic and pharmacodynamic (PK/PD) effects of (new) anti-diabetic drugs, in particular insulins. In a typical glucose clamp experiment, a drug-induced decline in blood glucose (BG) concentrations is prevented by infusing glucose with a variable glucose infusion rates (GIR) to keep blood glucose concentrations (BG) as closely as possible to a pre-defined target level.
- be more independent from subcontractors in order to ensure availability at all times
- have better control of the quality of the tubes for offering consistently high standards to our clients
- control the know-how of this core part of the ClampArt® system
- get the opportunity to optimise these tubes in different ways
so we can continue to offer our clients the best Clamp solutions.
The DLC mainly consists of two tubes with a small inner diameter that are connected together at one end in a Y-shape. One of the tubes at the Y-connector is linked to a small capillary, which can be placed into an intravenous cannula and therefore has direct contact to the blood in the vein. The blood is pumped through the tube of the DLC towards the device with the glucose sensor where the BG concentration is determined. These facts make the DLC itself a medical product.
 As a first step before starting the production of the DLC systems a risk analysis was performed to ensure that the DLC would be manufactured as a safe and reliable medical product. Because the DLC is used within an intravenous cannula, the DLC has to be a sterile medical product for single use only. To produce a sterile medical product Profil uses a two-step approach: The DLC is manufactured by trained associates in a new cleanroom at Profil. All critical components are purchased at certified manufactures in medical grade quality and all components need to pass a strict inspection upon arrival. This enables Profil to produce ultra-clean but not yet sterile DLC systems. In a second step these clean DLCs are sterilised in a validated ethylene oxide sterilisation process and are then ready to be used by Profil’s clinical staff for automated glucose clamps with the ClampArt® device.
As a first step before starting the production of the DLC systems a risk analysis was performed to ensure that the DLC would be manufactured as a safe and reliable medical product. Because the DLC is used within an intravenous cannula, the DLC has to be a sterile medical product for single use only. To produce a sterile medical product Profil uses a two-step approach: The DLC is manufactured by trained associates in a new cleanroom at Profil. All critical components are purchased at certified manufactures in medical grade quality and all components need to pass a strict inspection upon arrival. This enables Profil to produce ultra-clean but not yet sterile DLC systems. In a second step these clean DLCs are sterilised in a validated ethylene oxide sterilisation process and are then ready to be used by Profil’s clinical staff for automated glucose clamps with the ClampArt® device.
This entire manufacturing process, all documentation and validation including the implementation and maintenance of a full quality assurance system was inspected by a notified body and in August 2019 Profil obtained the EC-Certificate for a CE mark and subsequently to place the DLC tubing set on the market. For all future glucose clamp with the ClampArt® device Profil will use certified, self-manufactured DLC tubing sets.




