GLP-1 (glucagon-like peptide-1) receptor agonists have gained significant popularity due to their potential for weight loss. While some celebrities, such a tech billionaire Elon Musk [1], have amplified their reputation by using these drugs for weight management, it is crucial to examine their safety and efficacy for body weight loss. This article aims to provide an overview of GLP-1 based drugs, focusing on semaglutide (marketed as Ozempic® for type 2 diabetes and as Wegovy® for obesity) and tirzepatide (marketed as Mounjaro®), which is a dual GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 agonist. Some newer agents in development will also be discussed.
GLP-1 agonists exert their antidiabetic effects by lowering blood glucose levels through the stimulation of insulin release and inhibition of glucagon release. Additionally, these drugs have central effects that reduce appetite [2], although the exact mechanisms involved are not yet fully understood.
The following table presents the subcutaneous dosing regimens of approved GLP-1 and GLP-1/GIP agonists:
|
Brand name |
Active agent |
Dosing |
Approval |
Indication |
|
Ozempic® |
Semaglutide |
2 mg s.c. once weekly |
FDA 2017 EMA 2018
|
Type 2 diabetes mellitus |
|
Wegovy® |
Semaglutide |
2.4 mg s.c. once weekly |
FDA 2021 EMA 2022 |
Obesity or overweight in the presence of at least one weight-related comorbidity |
|
Mounjaro® |
Tirzepatide* |
Up to 15 mg s.c. once weekly |
FDA 2022 EMA 2022 |
Type 2 diabetes mellitus |
*Filed for obesity and overweight with the FDA in 2022 [3]
Originally, semaglutide was approved for the treatment of diabetes mellitus type 2 [4]. In spring 2021, a study [5] was published that led to its approval for the treatment of obesity with specific branding and dosing adjustments, see Table 1. The same year, tirzepatide gained a lot of attention after a study was published in The New England Journal of Medicine [6] that demonstrated its superior effect on weight loss compared to semaglutide in patients with type 2 diabetes, along with greater reduction in HbA1c levels.
While various studies have examined the effects of GLP-1 agonists in obesity, two notable phase 3 trials are worth highlighting in this context. The STEP 1 trial [7] included 1950 obese subjects receiving 2.4 mg of semaglutide versus placebo over 68 weeks, with a one-year extension. The SURMOUNT-1 trial [8] involved 2539 obese subjects receiving 5, 10, or 15 mg of tirzepatide versus placebo over 72 weeks.
Comparing these two trials, after 40 weeks, participants on semaglutide lost an average of 6.9 kg, whereas those on tirzepatide lost 7.8, 10.3, or 12.4 kg, respectively. Of course caution should always be exercised when making indirect comparisons, but also in a head-to-head comparison in a mode-of-action trial performed here at Profil, tirzepatide outperformed semaglutide. These findings demonstrate remarkable weight loss in obese individuals. However, there are some challenges in understanding the impact on body composition and the durability of the achieved weight loss.
Body composition
140 patients in the STEP 1 trial underwent DEXA scans for body composition analysis. Approximately 39% of the total weight loss observed in the STEP 1 trial was attributed to lean mass loss. Another study, SUSTAIN 8 [9], which utilized semaglutide for diabetes treatment, demonstrated an average loss of approximately 40% in lean mass.Durability
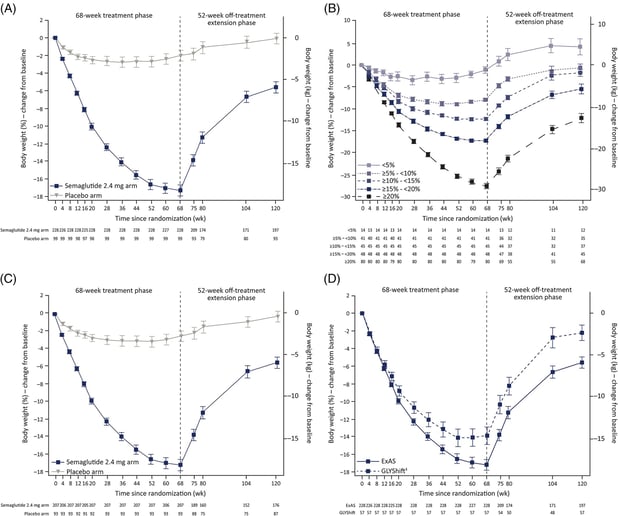
While the STEP 1 trial extension [10] revealed substantial weight loss after 68 weeks of semaglutide use, weight regain was observed upon discontinuation of the drug, see Figure 1. Notably, those who lost the most weight experienced the most significant regain. Although the extension lasted a full 120 weeks, it remains uncertain whether weight regain plateaus or continues until participants regain all or more of the lost weight.
What about side effects?
Known side effects of GLP-1 agonists were reported in 74.2% of subjects and primarily included gastrointestinal disorders such as nausea, diarrhea, vomiting, and constipation. These adverse effects were also the most common reasons for treatment discontinuation in the STEP 1 trial. Less frequent side effects observed in the trial included cholelithiasis and mild acute pancreatitis. Recently, the prescribing information [11] for Wegovy® included a warning regarding the risk of thyroid C-cell tumors, although its relevance to humans remains unknown.
GLP-1 agonists represent a significant advancement for patients with type 2 diabetes and obesity. However, it is crucial for healthcare professionals to thoroughly inform patients about the benefits and potential drawbacks of these drugs. It is concerning that young individuals, some of them who are merely overweight or not even that, are expressing interest in using GLP-1 agonists for weight loss. Consequently, physicians must exercise caution and carefully evaluate appropriate weight loss strategies for their patients.
Outlook
Numerous compounds currently in development hold great promise for type 2 diabetes and obesity. Among these, retatrutide, a GIP/GLP-1/glucagon receptor triagonist administered via weekly injections, made its debut at the recent 83rd Scientific Session of the American Diabetes Association. Notably, subjects with obesity who were treated with retatrutide [12] experienced a reduction of approximately 24% in total body weight, equating to 58 pounds. Impressively, this outcome appears to surpass the weight loss achieved with tirzepatide.
Similarly compelling is the emergence of survodutide [13], a novel compound acting as a dual agonist for both glucagon receptors and glucagon-like peptide 1 receptors. Administered on a weekly basis, survodutide elicited a substantial weight loss of up to 18.7% among overweight or obese individuals over a 46-week period. Furthermore, noteworthy insights were shared concerning oral GLP-1 receptor agonists. Orforglipron [14], an oral GLP-1 agonist, demonstrated significant weight loss after 36 weeks, demonstrating a remarkable 14.7% reduction in body weight. Notably, oral semaglutide [15] underwent evaluation at doses as high as 50 mg—an increase of over threefold compared to the presently available 14 mg dose—for the management of type 2 diabetes. Encouragingly, this intervention yielded a weight loss of 15.1%.
While further comprehensive data are indispensable to fully assess the potential of these compounds, the trajectory of this research appears highly promising for individuals grappling with overweight and obesity.
Read more about our expertise in testing medical technology: Insulin pump development and design, artificial pancreas study and the AP@home project, glucose monitoring.




