Introduction
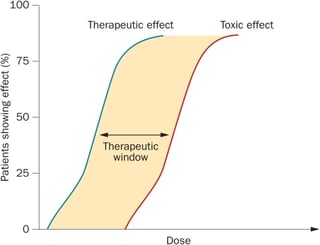
While drug-drug interactions are widely acknowledged and taken into account in clinical settings in patient management, the effects of food-drug interactions are not as well-known even though the effects may be just as harmful. For patients as well as medical doctors, it is important to consider various factors that can alternate the efficacy of a drug. During drug development, the pharmacokinetic and pharmacodynamic characteristics of a drug are investigated. The ideal therapeutic window of a drug, i.e. the range in which the drug is effective without being harmful, would have a minimum toxic concentration, which is multiple times the minimum effective concentration, offering a wide therapeutic window, free of side effects. This therapeutic window, or therapeutic index, can be quantified using the following formula:
Therapeutic index: LD50/ED50. LD50 stands for median lethal dose, ED50 stands for median effective dose
The therapeutic window of a drug is crucial for its adverse effects and toxicity. A drug overdose can occur due to the amount of the administered drug or due to metabolic factors that alternate the bioavailability of the drug, e.g. by inhibiting or inducing specific enzymes which metabolize the drug. Drugs with a narrow therapeutic window, i.e. drugs with therapeutic and toxic doses close together, need to be administered very carefully and often require close blood level monitoring. The efficacy of a drug is determined by the dosage itself and the circumstances under which the drug is being taken, e.g. time of the day, pre-/with/post- meal, etc. Therefore, the effects of a drug can be greatly multiplied or attenuated by various factors. These interactions, although complex, are of crucial importance for patients, medical doctors and investigators, who are planning and conducting clinical trials for the development of drugs. They may either lead to unintended toxic effects, or to not having a therapeutic effect at all. The following article will discuss the pharmacokinetic and the pharmacodynamic aspects of food-drug interactions and highlight a few major interactions.
Pharmacokinetics
In clinical pharmacology, pharmacokinetics (the metabolism of a drug), can be described using the so-called LADME scheme. LADME stands for liberation, absorption, distribution, metabolism, and excretion. The interactions can occur at any stage.
Liberation
Drugs can be administered through different routes, e.g. orally, intravenously, subcutaneously, topically, etc. However, most drugs are administered orally. From a pharmacological point of view, one of the main differences between the various routes of administration arises due to the bioavailability, i.e. the ratio of the amount of drug that reaches the systemic circulation to the amount given. While the bioavailability of an intravenously administered drug is as high as 100%, it can be as low as 0% for drugs administered otherwise. When taking a medication orally, it is essential that the drug passes successfully through the esophagus to the stomach. If a patient is lying down whilst taking the drug, there is a high chance that, instead of being swallowed all the way, it will stick to the esophagus and possibly causes ulcers in the tissue (note: especially patients taking bisphosphonates need to be cautious). Therefore, drugs should be taken in an upright position with at least 60 mL of water.
Absorption
Within the intestine, there are several confounding factors that can interfere with the absorption of a drug. First of all, should a drug be taken on an empty stomach? The interval between the meal and the intake of the drug can be a crucial factor for its efficacy. Enteric-coated drugs are designed to withstand the acidic pH levels within the stomach as the acid may disable the effects of the drug thus preventing the drug to be absorbed by the intestinal mucosa. However, if these drugs are taken shortly prior or after a meal, the pH in the stomach rises and the substances are released in the stomach where they may irritate the gastric mucosa and/or cause possible side effects. Taking food and drugs simultaneously may cause the drugs to dwell in the stomach for much longer as the stomach digests its contents and may possibly delay the uptake of the drug into the bloodstream. Drugs that are affected by this are, for example, NSAIDs or proton pump inhibitors. They should be taken 1-2 hours prior to a meal, together with a glass of water (note: the onset of action of NSAIDs has shown to be much higher when taken on an empty stomach. If long-term therapy with NSAIDs is required, please consult your primary physician, to avoid mucosal damage). Other factors that may interfere with the absorption of drugs include diets rich in fat or protein. Such diets may cause competitive inhibition of the absorption of a drug (e.g. L-DOPA). Besides foods, drinks may also interfere with the absorption of a drug. For example, products that are rich in calcium, such as mineral water, milk or dairy products can greatly reduce the bioavailability of drugs, such as bisphosphonates, tetracyclines, thyroid hormones and gyrase inhibitors. The substances may bind with the calcium and form a complex that cannot be absorbed through the intestine. Therefore, such products should not be consumed at the same time and the relevant drugs should be swallowed with a glass of low-calcium water. Among transport proteins in the intestinal cell membranes, there is a class called organic anion-transporting polypeptides, which actively transport certain antihypertensive drugs and H1-antihistamines. Ingredients of orange-, apple- and grapefruit juices can inhibit these polypeptides from being active for up to 2-4 hours, thus preventing the drugs from being absorbed. Therefore, these drugs should be taken with water at least 60 minutes prior to consumption of fruit-based beverages. Lastly, a compound called tannin, present in teas and coffees, forms a complex with iron supplements in the intestine, causing a reduction in its uptake. Iron supplements should be taken on an empty stomach, one hour prior to a meal and two hours prior to drinking tannin-containing beverages.
Distribution
The influence of food on the dispersion of drugs throughout the body is negligible compared to other drugs. Usually, drugs are bound to plasma proteins such as albumin, α1-acid glycoprotein, and lipoprotein. If multiple substances compete for the same binding site of a receptor, the drug with the higher affinity will displace others which may interfere with the drug’s efficacy. Prominent examples are coumarin anticoagulants and diclofenac, as they both have similar affinity to albumin, therefore leaving those patients, who are on coumarin anticoagulants together with diclofenac, at high risk of bleeding due to the increased concentration of free, bioavailable anticoagulant.
Metabolism
The goal of metabolism is the body’s detoxification of a substance to enable excretion of the metabolite. The liver plays the key role in the conversion of a substance into metabolites called biotransformation. Simply put, there are three phases to drug metabolism to enhance its excretion from the body, whether through the bile or urine. A process called ‘first-pass effect’ deactivates most drugs. Nevertheless, some drugs are activated through this process. Phase I reactions (consisting of oxidation, reduction and hydrolysis) in the liver are mainly executed by a giant enzyme complex called cytochrome P450. Phase II reactions conjugate the molecule to polar compounds by different enzymes. Phase III reactions can transform the compounds further. There are numerous families and subfamilies of CYP450. Approximately 60% of all drugs are metabolized by CYP3A4, a member of the CYP450 family. CYP3A4, which is found in the intestinal walls and in the liver, can be influenced by various types of foods, which either inhibit or induce its action. If a drug is deactivated by CYP3A4, induction of CYP3A4 leads to a decreased efficacy of the drug, and vice versa. A well-known inhibitor of intestinal CYP3A4 is grapefruit (juice). This inhibition lasts for 2-3 days until new mucosa cells have been regenerated. As the effect lasts for some time, when taking medication that is metabolized by CYP3A4, grapefruit (juice) must be avoided. Grapefruit (juice) increases the efficacy of drugs, such as certain calcium channel blockers, HMG-CoA reductase inhibitors i.e. statins, except for pravastatin, benzodiazepines, carbamazepine, some opioids, sildenafil and many others. It decreases the effect of aliskiren, celiprolol and fexofenadine, which are all substrates of organic anion-transporting polypeptides. For further details, see table below (please note that this list merely reflects a selection of substances).

Beverages containing caffeine (e.g. coffee, certain teas, coke, mate, energy drinks, yerba mate and dark chocolates) are metabolized by and serve as inhibitors of CYP1A2, which is the same enzyme that metabolizes clozapine and certain gyrase-inhibitors. If taken simultaneously, caffeine can enhance the drugs’ effects and side effects.
Excretion
The rate of elimination, i.e. the removal of the substance from the body, determines the half-life of a substance. Generally speaking, after four half-lives >90% of a substance is eliminated from the body. The organs responsible for excretion are the kidneys and the liver. Hydrophilic substances are renally excreted, whereas lipophilic (and some hydrophilic) substances are eliminated through the bile. Substances excreted renally are partly reabsorbed from the tubules into the capillaries. The process of reabsorption can be influenced by urinary pH. Alkaline urine (e.g. caused by a vegetarian diet), excretes acidic substances (e.g. barbiturates), more easily – a process which doctors make use of when patients are intoxicated with such substances. Special attention should be given to patients with hepatic or renal diseases, which lower their drug elimination rates and therefore increasing the risk of drug toxicity.
Pharmacodynamics
Pharmacodynamics describes the effects of a drug on the human body. As these effects differ greatly depending on the substance, few common examples are given below.
Vitamin K-rich foods
Coumarin-derived oral anticoagulants antagonize vitamin K, which is essential for the synthesis of clotting factors. Patients who are being treated with phenprocoumon or warfarin should be careful when it comes to foods rich in vitamin K, such as cauliflower, green tea, kale, spinach, liver, and many more. The consumption itself is not problematic, however, once a dose level of the anticoagulant has been established, vitamin K intake should remain consistent, otherwise patients are at increased risk of thromboembolism.
The ‘cheese effect’
Among other substances, monoamine oxidase inhibitors, especially the non-selective kind, irreversibly inhibit the deamination of tyramine, an amine that increases the release of norepinephrine. This causes vasoconstriction, which leads to increased blood pressure or even hypertensive crisis. As high amounts of tyramine can be found in red wine and cheese, this is called the ‘cheese-effect’.
Alcohol
Overall, mixing alcohol and drugs can increase the risk of complications greatly. Drugs that inhibit aldehyde dehydrogenase (e.g. sulfonylurea and metronidazole) for example can increase the effect of alcohol by slowing down its metabolism. Other drugs, such as methotrexate or isoniazid, increase the risk of liver damage if alcohol is consumed regularly. Patients with diabetes mellitus should consume alcohol with care as it inhibits the hepatic gluconeogenesis, which leads to low blood sugar and may even cause hypoglycaemia. If the patient is taking metformin, alcohol can increase the risk of lactic acidosis greatly. Therefore, with moderate alcohol consumption, a sufficient carbohydrate intake is recommended for these patients.
Conclusion
Food-drug interactions are extremely complex. Many factors must be taken into consideration, e.g. the compounds of the substrate, confounders of the uptake, distribution and elimination of the drug, enhancers/inducers of its metabolism and the underlying disease of the individual that may interfere with all of these processes. As a patient, it is very important to pay close attention to the instructions on how to take their medications. For medical doctors, one should always be aware of the pharmacokinetic and pharmacodynamics characteristics of a drug, as well as the patient’s medical history, in order to be able give the correct and tailored instructions on taking the medications. As a medical researcher, it is essential that all of the above must be considered when designing and conducting clinical trials.




