The euglycemic clamp technique has been used as a standard method for assessing time action profiles of insulins for decades. In recent years, new challenges have emerged for investigators performing glucose clamp trials. These are driven by two opposing trends in the development of novel insulins: On the one hand, drug developers are formulating insulin products with a very rapid onset of action and a short duration of action – often referred to as “ultra-rapid insulins” [1,2]. On the other hand, novel basal insulins are being developed with an “ultra-long” duration of action with flatter activity profiles than previously available products [3,4,5].

Clamping ultra-rapid insulin is tricky
In an earlier blog post we discussed challenges of studying time-action profiles of ultra-rapid insulins with the glucose clamp technique. In brief, the steep and sudden increase and decrease in the pharmacologic activity profiles of rapid-acting insulins often pose a challenge for investigators. Blood glucose changes must be closely monitored and glucose infusion rates have to be adapted fast and frequently in order to maintain blood glucose at the target level. Technical progress in the field of automated clamp procedures has enabled the accurate and continuous monitoring of blood glucose levels with little lag time. In addition, new algorithms for automated adjustment of the glucose infusion rate (or “GIR”) have been implemented to further reduce oscillations of the blood glucose and keep it closer to target. Both aspects are a prerequisite for reaching optimized clamp quality in terms of accuracy and patients’ safety [6,7].
Clamp studies in ultra-long-acting insulins should be easy, right?
At first glance, assessing the activity profiles of an ultra-long-acting insulins seems to be an easy task when compared to any (ultra-)rapid-acting insulin. Obviously, there will be no sudden changes of activity that require quick and accurate decisions on GIR adjustments. All in all, the smooth and flat shape of the profile should make it relatively easy to maintain the blood glucose at the target with only minor adjustments of glucose infusion rates.
Well, from a technical standpoint, this view is at least somewhat correct. Still, there are a number of pitfalls to consider when doing glucose clamp studies with basal insulins. In terms of study design interpretation of data, these studies are more demanding than for rapid-acting products. The following are a few examples for typical challenges that investigators are faced with:
Challenge number 1: You cannot clamp this long…
Glucose clamp are ideally designed to cover the entire duration of action of an insulin dose and yield a complete activity profile from onset to peak activity and further on until end of action. Available ultra-long insulin products such as insulin degludec have a duration of action of more than 40 hours of activity. Future developments may even target longer durations of action, and could for example lead to the development of “once-weekly” basal insulins, similar to the developments seen for GLP-1-analogs [8,9].
The maximum duration of a clamp procedure is, however, limited, mainly due to the strain that is put on people who are undergoing a glucose clamp procedure: First, there is the need to remain fasted from several hours prior to the clamp and throughout the duration of procedure. Next, study participants have to adhere to bed rest. Moreover, the blood loss required for frequent or continuous blood glucose monitoring (often in addtion to blood volume for pharmacokinetic testing) has to be taken into consideration and may limit the acceptable total glucose clamp time for an individual participant. Glucose clamps are therefore rarely done for periods beyond approximately 36 hours (including several hours of “run-in time” before dosing for blood glucose stabilization). This obviously makes it difficult to fully characterize any basal insulin with a duration of action of 40 hours or beyond.
… but you can clamp more frequently…
A common approach is to characterize activity during one dosing interval (usually 24 hours) rather than more extended periods. In a single dose study this will fail to cover the full duration of action. If done after repeated doses this approach is useful to characterize the profile once steady state conditions have been reached. Moreover, a series of intermittent clamps during the first days of daily treatment can be helpful to investigate how fast the insulin effect builds up towards steady state. This information can be used to develop titration regimens for ultra-Iong-acting insulin [10].
In theory, a similar approach could be applied for insulins with even longer activity and less frequent dosing intervals. For example, an insulin designed for once weekly administration could be characterized by a series of relatively short clamp experiments that should be scheduled to cover the activity during early and late part of the activity profile, and during the expected time of peak activity.
Still, there will be a number of limitations: This approach might be prone to be biased by circadianic and day-to-day variability of insulin sensitivity. Therefore, potential bias must be minimized by standardizing potential confounders such as physical activity, food intake and insulin treatment between the clamps.
Challenge number 2: A perfect dose in life is not a perfect dose in a glucose clamp study
Rapid-acting insulins can be used in glucose clamp studies over a very wide range of doses, covering the entire range of clinically relevant doses. A thoroughly conducted glucose clamp can safely counteract insulin activity of doses which are above a patient’s therapeutic range and – on the other hand – the clamp method is sensitive enough to detect effects of quite low doses as long as they result in a short but measurable increase of glucose infusion rate. Unfortunately this does not hold true when it comes to basal insulins.
If a patient with diabetes who is already treated with insulin is given his or her usual basal insulin dose under glucose clamp conditions, this will usually not result in any significant and consistent glucose infusion rate. In real-life this “optimal dose” of basal insulin will simply keep blood glucose levels at euglycemia without any need for additional food consumption to avoid hypoglycaemia. In a clamp setting the “real-life optimal dose” will keep blood glucose levels constant at euglycaemia without the need for any glucose infusion. In other words, in order to produce meaningful results during a clamp experiment, glucose clamps with long-acting insulins should investigate relatively high doses, which elicit a robust glucose infusion rate (the measurable effect) [11].
Therefore, the typical recommended dose range for basal insulins for clamp experiments is between 0.4 U/kg and 0.6 U/kg [12].
Though doses below 0.4 U/kg are actually used by many patients and are therefore certainly of clinical relevance, these doses are not well suited for a clamp study. The lower the chosen doses are, the higher the number of “low responders” or “non responders” (in terms of glucose infusion rate) will be. Apart from the insufficient GIR repsonse, these patients will often show blood glucose levels rising above target during the clamp. This will increase the total variabilty for GIR related parameters and thereby reduce the overall power of the study.
Challenge number 3: Early glucose escape
Another problem can result from the low early activity of basal insulins and their late onset of action.
For rapid-acting insulins, the onset of action can be assessed in an easy and straightforward way: If the study is done in people with type 1 diabetes, blood glucose is usually lowered before dosing by means of intravenous insulin, while avoiding any GIR. This approach will ensure that the insulin infusion rates are kept as low as possible. To avoid any carry-over effects, intravenous insulin is usually completely terminated from 30 minutes before dosing. The remaining insulin action from the intravenous infusion is sufficient to control the subject’s BG until dosing, while any activity after dosing is neglible due to the short half-life of iv insulin. After dosing, GIR will be started as soon as glucose has dropped to a predefined level below target. This timepoint is often a study endpoint defined as the onset of action.
For basal insulins, estimating the onset of action or – more generally speaking - early insulin activity is more difficult, because of the long time it may take from dosing until any substantial blood glucose lowering effect emerges. If the insulin infusion is stopped before dosing, as it is usually done in studies with rapid-acting insulins, blood glucose levels may initially rise post-dosing and they may keep going up to levels above target for several hours. This may even result in an early termination of the clamp due to hyperglycemic levels before onset of action has even occurred. But even less extreme cases of “early blood glucose escape” are certainly undesireable because they will impact the variability of glucose infusion rate and will result in an underestimation of the initial PD activity (See Figure 1) [13].
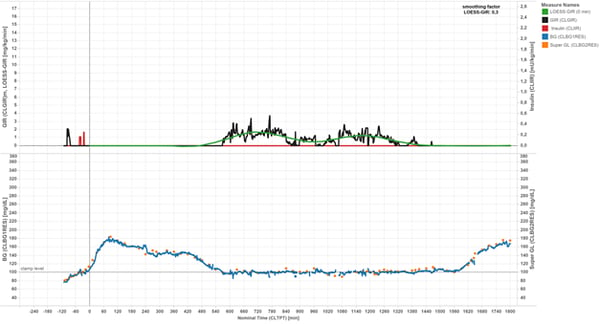 Figure 1: Potential bias by low early insulin activity: Shortly after dosing with insulin glargine, blood glucose levels of this person with type 1 diabetes rise to hyperglycemic levels while insulin activity is still low. Blood glucose starts dropping from approx. 90 minutes post dosing, however, this apparent effect of the tested insulin does not lead to any glucose infusion as long as blood glucose has not yet returned to the target level. The glucose infusion rate curve may therefore lead to an underestimation of the early pharmacodynamic activity.
Figure 1: Potential bias by low early insulin activity: Shortly after dosing with insulin glargine, blood glucose levels of this person with type 1 diabetes rise to hyperglycemic levels while insulin activity is still low. Blood glucose starts dropping from approx. 90 minutes post dosing, however, this apparent effect of the tested insulin does not lead to any glucose infusion as long as blood glucose has not yet returned to the target level. The glucose infusion rate curve may therefore lead to an underestimation of the early pharmacodynamic activity.
It has therefore been proposed to continue intravenous insulin infusion post-dosing and to gradually taper off insulin rates. However, this approach has some disadvantages. Firstly, carry-over effects and interference with the study insulin (at least for PD and possibly also PK) cannot be fully avoided. Moreover, there is no clear agreement on how to adapt the insulin infusion rates and to reliably maintain euglycemia for hours only by adjusting insulin infusion only. Confounding factors such as variability of glucose measurements or even physiological BG fluctuations can lead to a misinterpretation of the early activity and action, and lead to an inadequately early start of glucose infusion.
Switching to a study population of healthy people could be another viable option to avoid this situation. Though this would eliminate any blood glucose escapes and though GIR parameters are often lower, study results can easily be biased by the presence of endogenous insulin. Therefore, C-peptide levels must be monitored and thresholds should be defined for an exclusion of PD data. But even if C-peptide levels remain relatively low, the small pharmacodynamic effects expected during the early and late parts of the time action profile are still prone to bias.
There is no general agreement on how to address this challenge. Of note, variability of GIR will be largely reduced in any clamp study investigating steady state doses due to carry-over effects after multiple doses, and other than for rapid-acting insulins, parameters of early insulin activity are usually not any main endpoint of studies with basal insulin. Investigators should nevertheless be fully aware of the limitations of the glucose clamp method when interpreting the effects of low basal insulin doses, and blood glucose curves should always be interpreted together with the glucose infusion rate curves to fully understand the validity of an insulin time-action profile.




