The CONCEPTT trial
Pregnant women with type 1 diabetes have an increased risk of preeclampsia, polyhydramnios and caesarean sections. Furthermore, for the infants of pregnant women with type 1 diabetes increased rates of congenital anomalies, premature deliveries, macrosomia, stillbirth and NICU admissions are reported. All these risks are known to increase with higher HbA1c values before or during pregnancy. Therefore the use of continuous monitoring in such patients might be advantageous, aiming for an optimal glucose control during pregnancy.
This multicentre, open-label, randomised controlled trial investigated in two parallel trials the effect of CGM use on the primary outcome (difference in change in HbA1c) in pregnant women or women planning to become pregnant. The two parallel trials were performed in a) pregnant women (≤ 13 weeks and 6 Gestational Days up to 34 weeks of gestation) and b) in women planning pregnancy. In both groups the women were randomised (1:1) to either the use of CGM in addition to their usual therapy or using their usual therapy without CGM.
An overview of the distribution of the participants is shown in the following flow charts (A: pregnant women, B: women planning pregnancy):
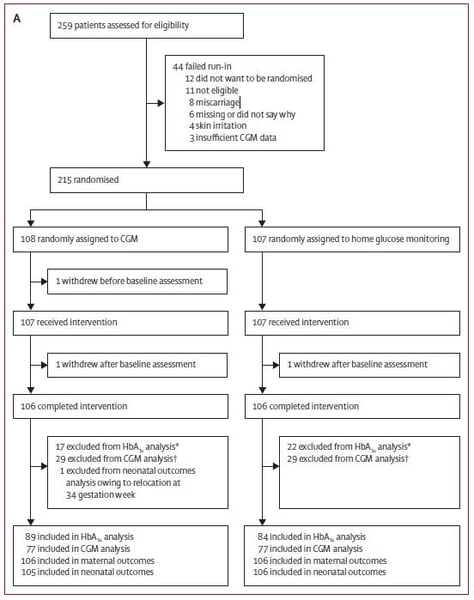 Figure 1: Distribution of the participants: pregnant women
Figure 1: Distribution of the participants: pregnant women
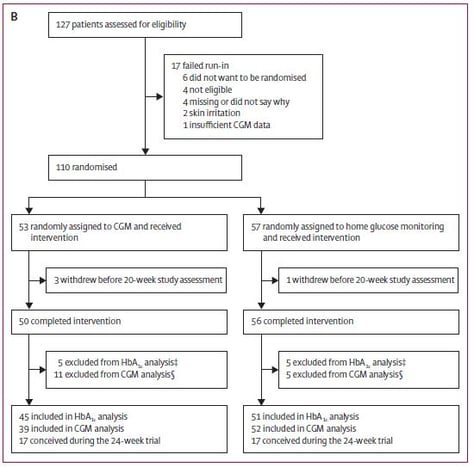
Figure 2: Distribution of the participants: women planning pregnancy
For the trial 386 participants were assessed for eligibility between March 25th, 2013 and March 22nd, 2016; 325 participants were randomised, 215 pregnant women and 110 women planning pregnancy. The difference in change of HbA1c was measured between randomisation to 34 weeks gestation in the pregnant women and from inclusion to 24 weeks in the women planning pregnancy. Secondary outcomes were time spent in, above or below the recommended glucose control target range (63 mg/dl – 140 mg/dl), area under the curve for glucose levels, episodes of hypoglycemia and glucose variability measures derived from CGM measures. For the infants the following parameters were predefined to be evaluated:
-
preterm delivery
-
neonatal hypoglycemia requiring iv dextrose
-
neonatal intensive care unit admission (at least 24h)
-
cord blood gas pH
-
total length of hospital stay
-
birthweight
-
macrosomia
For the present purpose only the results for the pregnant women trial will be presented.
In the primary analysis of the trial a small but significant between-group difference was found for the change in HbA1c from baseline to 34 weeks’ gestation with an advantage for CGM (mean difference -0.19%, 95% CI -0.34 to -0.03; p 0 0.0207) (Figure 3). Adjustment for baseline covariates (e.g. maternal education, smoking, BMI, duration of diabetes and episodes of severe hypoglycemia) did not change the effect.
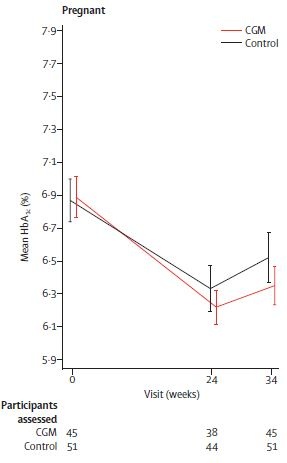 Figure 3: Primary glycemic outcome assessed by HbA1c
Figure 3: Primary glycemic outcome assessed by HbA1c
Moreover, within the group of pregnant women using CGM, more time was spent in the recommended glucose control target range of 3.5 to 7.8 mmol/L and reduced time above target in comparison to the control group. The mean amplitudes of glucose excursions were reduced as well.
No between-group difference was found with regards to subjective assessments measured by Blood Glucose Monitoring System Ranking Questionnaire (BGMSSRQ), Problem Areas in Diabetes (PAID), Short-Form-12 (assessing the general health status of the subject) and Hypoglycemia Fear Survey (HFS II).
More interesting were the neonatal outcomes. The most significant findings in the offspring of pregnant women being in the CGM group were a reduced total length of hospital stay (p = 0.0091), Figure 4 and a decreased proportion of neonates large for gestational age (p = 0.0210).
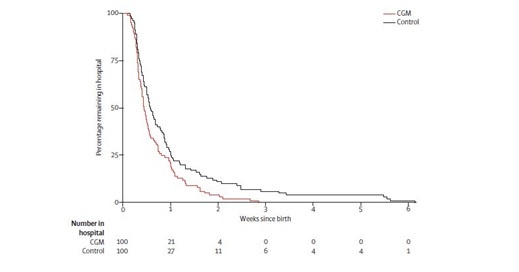 Figure 4: Kaplan-Meier plot showing infants’ length of hospital stay
Figure 4: Kaplan-Meier plot showing infants’ length of hospital stay
A similar difference (p = 0.0250) was found for neonatal hypoglycemia requiring iv dextrose; in the CGM group 15 infants required iv dextrose whereas in the control group 28 had to be treated in this way.
Most common adverse events were skin reactions in both groups, however in the CGM group 48% were affected, whereas only 8% were affected in the control group.
Conclusion
In conclusion the study shows that the use of CGM during pregnancy in patients with type 1 diabetes is associated with improved neonatal outcome. It could be demonstrated that the direct CGM measures of day-to-day exposure to maternal hyperglycemia are more relevant for neonatal outcomes than the surrogate marker HbA1c.
References
Feig DS, Donovan LE, Corcoy R, Murphy KE et al.: Lancet. 2017 Nov 25;390(10110):2347-2359.




