A pathophysiological approach in the treatment of patients with diabetes mellitus
Like any drug development process, developing novel anti-diabetic drugs is a difficult endeavour. This holds especially true in the midst of the wave of novel drugs and drug classes that we have seen entering the market over the last years. To be successful in this area, it is crucial to think beyond blood glucose levels when developing novel compounds.
Insulin resistance, alpha-, and beta cell dysfunction characterize the pathophysiological triade in the development of type 2 diabetes mellitus. Investigation of drug effects and/or drug interactions with these components in the development and progression of diabetes mellitus can provide more rationality for the selection of drugs or drug combinations in the treatment of the disease. To address the importance of islets of langerhans in diabetes and their complicated balance, this text deals with the islets of Langerhans and the alpha- and beta cell function in diabetes. It then shifts focus to the pharmacological intervention and methods to study the cells' function to enable developers choose the right tools for their clinical trials in this field.
Blood glucose and the role of the Islet of Langerhans
In individuals without diabetes, blood glucose is kept within a narrow range by a complex interaction of several regulatory pathways. In patients with diabetes mellitus, these well balanced signalling pathways become unsettled with a loss of blood glucose control. The islet of Langerhans play a crucial role in the pathogenesis of type 1 and type 2 diabetes mellitus. The human island of Langerhans cover alpha-, beta-, delta-, and pancreatic polypeptide (pp) secreting cells. The cytoarchitecture of the human islet, where the beta cells show close associations with the other endocrine islet cells, suggest comprehensive paracrine interactions. Islets are densely vascularised and the islet cells are exposed to changes in the arterial milieu, like insulin fluctuations. Insulin derived from the beta cell, as well as glucagon derived from the alpha cell, are drained in to the portal circulation where they reach the liver tissue. In the liver tissue, the insulin/glucagon ratio controls for hepatic gluconeogenesis, glycogenesis, and glycogenolysis. In the fasting state, basal levels of glucagon account for up to 70% of circulating glucose levels (1). As illustrated in figure 1, rising blood glucose levels increase the release of insulin from the beta cell and supress glucagon secretion from the alpha cell, and vice versa.
By changing the ratio of glucagon to insulin within the portal circulation, alpha- and beta cell activity accounts for hepatic glucose release during low blood glucose concentrations and hepatic glucose uptake in case of high blood glucose concentrations.
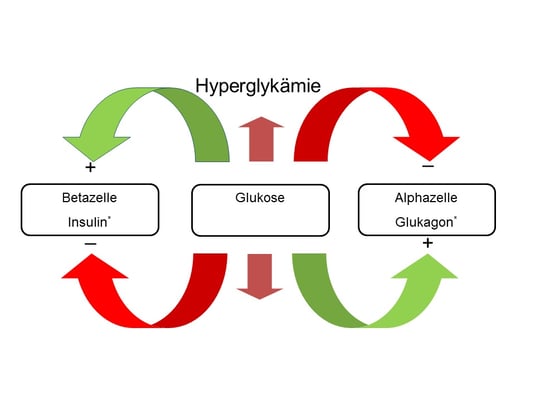
Alpha cells express a high number of insulin receptors on their surface, and paracrine increments in insulin concentrations within the pancreatic islets suppress the release of glucagon from the alpha cell. In this context, it is remarkable that alpha cells are exposed to 100 times more insulin than peripheral tissues. Interestingly, recent data suggest that insulin may also regulate glucagon secretion through actions in the ventromedial hypothalamus and the activation of autonomic pathways (2,3). In the opposite way, declining blood glucose concentrations cause a decrease in the local intra-islet insulin concentration, and thereby an unrestrained release of glucagon from the alpha cells (4,5). Elimination of the insulin receptor from pancreatic alpha cells was shown to abolish the glucagon response to low glucose concentrations (6), and a couple of recent investigations found that a decline in intra-islet insulin concentration is important to trigger an appropriate glucagon response to hypoglycaemia. Several factors are known to modulate the secretory response of alpha and beta cells, such as gastrointestinal hormones (7,8), catecholamines (9,10), local Zn2+ concentration (11,12), and the autonomic nervous system (13,14).
Alpha- and beta cell function in diabetes mellitus type 1
In type 1 diabetes mellitus, autoimmune destruction of the beta cells leads to the development of an absolute insulin deficiency. In this case, alpha cells comprise approximately 75% of the total islet cell mass (15). In parallel, the glucagon levels increase in patients with type 1 diabetes most probably due to a loss of paracrine suppression from intra-islet insulin. Elevated glucagon levels in patients with type 1 diabetes are supposed to count for insulin resistance and the difficulties to achieve adequate blood glucose control. An elevated glucagon/insulin ratio has been shown to accelerate gluconeogenesis and fatty acid oxidation leading to the formation of ketone bodies (16), most probably contributing to an increased risk of ketonemia in patients with type 1 diabetes mellitus. While glucagon levels in patients with type 1 diabetes are elevated, no appropriate increase in glucagon appears in response to low blood glucose levels (17). The missing counter regulatory response in type 1 diabetes mellitus is explained by the lack of an intra-islet drop in insulin concentration, keeping off an appropriate signalling for the alpha cell to increase glucagon secretion (17-19). The missing “intra-islet switch off signal” is a major driver of the increased risk for severe hypoglycaemia in patients with type 1 diabetes. Subcutaneous application of insulin in patients with diabetes mellitus causes a misallocation in the insulin distribution, with high insulin levels in peripheral tissues and an inappropriate glucagon / insulin ratio in the portal circulation.
Alpha- and beta cell function in diabetes mellitus type 2
Type 2 Diabetes mellitus (T2DM) is a complex disease, characterized by insulin resistance, followed by declining beta cell function and a disrupted glucagon-insulin balance. Disturbed pro-insulin processing with declining insulin levels and an elevation in fasting and postprandial glucagon levels characterize the development and progression of T2DM (20-22). An increase in the precursor molecule of insulin, intact proinsulin, was identified as a potent marker for the prediction of beta cell failure and often precedes the development of type 2 diabetes mellitus (23,24). Numerous studies suggested an association between increased intact proinsulin levels and the development of cardiovascular complications in subjects with or without diabetes (25-30). Even though the exact mechanism how proinsulin might be involved in the pathogenesis of atherogenesis is not fully understood, there is increasing evidence that proinsulin raises plasminogen activator inhibitor-1 (PAI-1) levels (31-33), thereby accelerating the high pro-thrombotic potency observed in patients with T2DM. In addition, proinsulin was found to bind with high affinity to the insulin-receptor-A-isoform leading to a pronounced activation of the ERK/p70S6K downstream signalling cascade (34), thereby, accelerating the mitogenic and atherogenic signalling of the insulin receptor.
A large body of evidence implicates hyperglucagonemia in the maintenance of increased rates of hepatic glucose output in T2DM (35,36). Under fasting conditions hyperglucagonemia sustains glucose overproduction, and impaired glucagon suppression after a meal contributes to the postprandial hyperglycemia in T2DM (21,37). Elevated fasting and postprandial glucagon levels substantially contribute to insulin resistance and decrease the efficacy of antidiabetic drugs, including treatment with exogeneous insulin (38-40). Beside the mismatch in islet cell functionality, increased beta cell apoptosis and a proliferation of alpha cells characterize islet cell remodelling in patients with type 2 diabetes mellitus (41).
Pharmacological effects on alpha- and beta cell function
Various new pharmacological approaches have been introduced in the treatment of diabetes mellitus. Other new mechanistic pathways to treat patients with T2DM are the topic of ongoing preclinical and clinical research. Some of these therapeutic strategies directly address glucagon specific pathways (20,42). Therefore, the armamentarium for pharmacological interventions in patients with diabetes mellitus is growing consistently. This implies an increasing need to understand the effects of different pharmacological interventions on the pathophysiology of the disease beyond their obvious effects on blood glucose levels.
While metformin and glitazones affect insulin sensitivity, sulfonylureas and GLP-1 based treatments interact with alpha and/or beta cell activity. It seems noteworthy that sulfonylureas and GLP-1 based treatments regulate blood glucose values by totally different effects on alpha and beta cell function. In the post-absorptive state, sulfonylureas predominantly augment the release of insulin from the beta cell, leading to an expeditious exhaustion of the beta cell as indicated by an immediate increase in the release of proinsulin and an increase in the rate of apoptosis (43,44). In case of low blood glucose values, sulfonylureas still evolve their stimulatory effects on the beta cell and inhibit the release of glucagon from the alpha cell, driving protracted hypoglycemic episodes in patients with T2DM (45). Interestingly, sulfonylureas augment the glucagon secretion from the alpha cell in patients with T1DM, most probably due to the absence of beta cells within the pancreatic islet (46,47). In contrast to sulfonylureas, treatment with DPP-IV inhibitors were shown to reduce the postprandial release of glucagon from the alpha cell, thereby taking of the postprandial workload from the beta cell with a subsequent fall of proinsulin concentrations in the blood (48). Therefore, sulfonylureas control postprandial glucose values by stimulating the release of insulin from the beta cell, while DPP-IV inhibitors reduce postprandial release of glucagon from the alpha cell with a subsequent relief of strain from the beta cell. DPP-IV inhibitors enhance alpha cell responsiveness to both the suppressive effect of hyperglycemia and the stimulatory effect of hypoglycemia (49).
SGLT-2 inhibitors, a new class of antidiabetic agents reduce blood glucose by increasing glucose excretion through the kidneys. Interestingly, recent investigations revealed an increase in endogenous glucose production following treatment with SGLT-2 inhibitors, most probably caused by an increase in plasma glucagon levels (50,51). The underlying molecular pathways causing the counter-regulators increase in alpha cell activity are topic of numerous scientific discussions. There is increasing evidence that the combination of SGLT-2 inhibition and DPP-IV inhibition evolves additive effects on blood glucose control with a restoration of overall islet cell physiology (41).
Assessing insulin resistance, alpha-, and beta cell function in diabetes mellitus
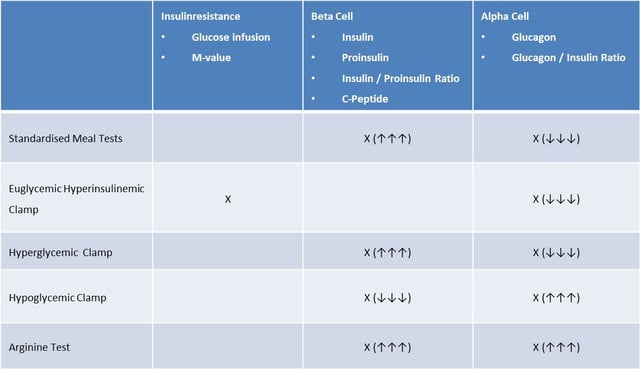
There are numerous options to investigate the different components in the pathophysiology of T2DM (20,36). As shown in the table below, standardised clamp procedures might be helpful to evaluate insulin resistance, alpha and beta cell function in patients with T2DM. A standardised meal test or a hyperglycemic clamp can provide information on the capacity of the beta cell to respond to increasing blood glucose concentrations. The additional measurement of proinsulin during increasing blood glucose levels will provide further information about the functional capacity of the beta cell. Additional measurement of glucagon provides information about the alpha- and beta cell interaction and a sufficient suppression of the alpha cells under hyperglycemic conditions. Measurement of the glucose infusion rate (M-value) and glucagon levels within a euglycemic-hyperinsulinemic clamp allows the characterization of insulin resistance and alpha cell responsiveness. In contrast, a stepwise hypoglycemic clamp can be used for the evaluation of alpha- and beta cell function during low blood glucose levels providing important information about the counter-regulatory capacity during treatment with different antidiabetic treatments. The different clamp investigations can be complemented by a pharmacological stimulation of the alpha- and beta cells with arginine to obtain information about the overall functional capacity of the alpha- and beta cells.
Conclusion
In conclusion, insulin resistance, alpha-, and beta cell dysfunction characterize the pathophysiological Triade in the development of type 2 diabetes mellitus. Investigation of drug effects and/or drug interactions with these components in the development and progression of diabetes mellitus can provide more rationality for the selection of drugs or drug combinations in the treatment of the disease.
If you don't want to miss similar posts on interesting topics, then sign up to our monthly blog newsletter on this page.
Reference List
1. Cherrington AD, Liljenquist JE, Shulman GI, Williams PE, Lacy WW: Importance of hypoglycemia-induced glucose production during isolated glucagon deficiency. Am J Physiol 236:E263-E271, 1979
2. Paranjape SA, Chan O, Zhu W, Horblitt AM, Grillo CA, Wilson S, Reagan L, Sherwin RS: Chronic reduction of insulin receptors in the ventromedial hypothalamus produces glucose intolerance and islet dysfunction in the absence of weight gain. Am J Physiol Endocrinol Metab 301:E978-E983, 2011
3. Paranjape SA, Chan O, Zhu W, Horblitt AM, McNay EC, Cresswell JA, Bogan JS, McCrimmon RJ, Sherwin RS: Influence of insulin in the ventromedial hypothalamus on pancreatic glucagon secretion in vivo. Diabetes 59:1521-1527, 2010
4. Hope KM, Tran PO, Zhou H, Oseid E, Leroy E, Robertson RP: Regulation of alpha-cell function by the beta-cell in isolated human and rat islets deprived of glucose: the "switch-off" hypothesis. Diabetes 53:1488-1495, 2004
5. Meier JJ, Kjems LL, Veldhuis JD, Lefebvre P, Butler PC: Postprandial suppression of glucagon secretion depends on intact pulsatile insulin secretion: further evidence for the intraislet insulin hypothesis. Diabetes 55:1051-1056, 2006
6. Diao J, Asghar Z, Chan CB, Wheeler MB: Glucose-regulated glucagon secretion requires insulin receptor expression in pancreatic alpha-cells. J Biol Chem 280:33487-33496, 2005
7. Hompesch M, Jones-Leone A, Carr MC, Matthews J, Zhi H, Young M, Morrow L, Reinhardt RR: Albiglutide does not impair the counter-regulatory hormone response to hypoglycaemia: a randomized, double-blind, placebo-controlled, stepped glucose clamp study in subjects with type 2 diabetes mellitus. Diabetes Obes Metab 17:82-90, 2015
8. Degn KB, Brock B, Juhl CB, Djurhuus CB, Grubert J, Kim D, Han J, Taylor K, Fineman M, Schmitz O: Effect of intravenous infusion of exenatide (synthetic exendin-4) on glucose-dependent insulin secretion and counterregulation during hypoglycemia. Diabetes 53:2397-2403, 2004
9. Gerich JE, Karam JH, Forsham PH: Stimulation of glucagon secretion by epinephrine in man. J Clin Endocrinol Metab 37:479-481, 1973
10. Schuit FC, Pipeleers DG: Differences in adrenergic recognition by pancreatic A and B cells. Science 232:875-877, 1986
11. Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB: Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54:1808-1815, 2005
12. Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB: Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol 5:330-335, 2003
13. Havel PJ, Ahren B: Activation of autonomic nerves and the adrenal medulla contributes to increased glucagon secretion during moderate insulin-induced hypoglycemia in women. Diabetes 46:801-807, 1997
14. Ahren B: Autonomic regulation of islet hormone secretion--implications for health and disease. Int J Obes Relat Metab Disord 43:393-410, 2000
15. Orci L, Baetens D, Rufener C, Amherdt M, Ravazzola M, Studer P, Malaisse-Lagae F, Unger RH: Hypertrophy and hyperplasia of somatostatin-containing D-cells in diabetes. Proc Natl Acad Sci U S A 73:1338-1342, 1976
16. Vons C, Pegorier JP, Girard J, Kohl C, Ivanov MA, Franco D: Regulation of fatty-acid metabolism by pancreatic hormones in cultured human hepatocytes. Hepatology 13:1126-1130, 1991
17. Cryer PE, Fisher JN, Shamoon H: Hypoglycemia. Diabetes Care 17:734-755, 1994
18. Clarke WL, Gonder-Frederick LA, Richards FE, Cryer PE: Multifactorial origin of hypoglycemic symptom unawareness in IDDM. Association with defective glucose counterregulation and better glycemic control. Diabetes 40:680-685, 1991
19. Hope KM, Tran PO, Zhou H, Oseid E, Leroy E, Robertson RP: Regulation of alpha-cell function by the beta-cell in isolated human and rat islets deprived of glucose: the "switch-off" hypothesis. Diabetes 53:1488-1495, 2004
20. Unger RH, Cherrington AD: Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 122:4-12, 2012
21. Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA: Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 85:4053-4059, 2000
22. Muller WA, Faloona GR, Aguilar-Parada E, Unger RH: Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med 283:109-115, 1970
23. Pradhan AD, Manson JE, Meigs JB, Rifai N, Buring JE, Liu S, Ridker PM: Insulin, proinsulin, proinsulin:insulin ratio, and the risk of developing type 2 diabetes mellitus in women. Am J Med 114:438-444, 2003
24. Hanley AJ, D'Agostino R, Jr., Wagenknecht LE, Saad MF, Savage PJ, Bergman R, Haffner SM: Increased proinsulin levels and decreased acute insulin response independently predict the incidence of type 2 diabetes in the insulin resistance atherosclerosis study. Diabetes 51:1263-1270, 2002
25. Alssema M, Dekker JM, Nijpels G, Stehouwer CD, Bouter LM, Heine RJ: Proinsulin concentration is an independent predictor of all-cause and cardiovascular mortality: an 11-year follow-up of the Hoorn Study. Diabetes Care 28:860-865, 2005
26. Oh JY, Barrett-Connor E, Wedick NM: Sex differences in the association between proinsulin and intact insulin with coronary heart disease in nondiabetic older adults: the Rancho Bernardo Study. Circulation 105:1311-1316, 2002
27. Wohlin M, Sundstrom J, Arnlov J, Andren B, Zethelius B, Lind L: Impaired insulin sensitivity is an independent predictor of common carotid intima-media thickness in a population sample of elderly men. Atherosclerosis 170:181-185, 2003
28. Zethelius B, Lithell H, Hales CN, Berne C: Insulin sensitivity, proinsulin and insulin as predictors of coronary heart disease. A population-based 10-year, follow-up study in 70-year old men using the euglycaemic insulin clamp. Int J Obes Relat Metab Disord 48:862-867, 2005
29. Zethelius B, Byberg L, Hales CN, Lithell H, Berne C: Proinsulin is an independent predictor of coronary heart disease: Report from a 27-year follow-up study. Circulation 105:2153-2158, 2002
30. Wiberg B, Sundstrom J, Zethelius B, Lind L: Insulin sensitivity measured by the euglycaemic insulin clamp and proinsulin levels as predictors of stroke in elderly men. Int J Obes Relat Metab Disord 52:90-96, 2009
31. Nordt TK, Bode C, Sobel BE: Stimulation in vivo of expression of intra-abdominal adipose tissue plasminogen activator inhibitor Type I by proinsulin. Int J Obes Relat Metab Disord 44:1121-1124, 2001
32. Festa A, D'Agostino R, Jr., Mykkanen L, Tracy RP, Zaccaro DJ, Hales CN, Haffner SM: Relative contribution of insulin and its precursors to fibrinogen and PAI-1 in a large population with different states of glucose tolerance. The Insulin Resistance Atherosclerosis Study (IRAS). Arterioscler Thromb Vasc Biol 19:562-568, 1999
33. Lyon CJ, Hsueh WA: Effect of plasminogen activator inhibitor-1 in diabetes mellitus and cardiovascular disease. Am J Med 115 Suppl 8A:62S-68S, 2003
34. Malaguarnera R, Sacco A, Voci C, Pandini G, Vigneri R, Belfiore A: Proinsulin binds with high affinity the insulin receptor isoform A and predominantly activates the mitogenic pathway. Endocrinology 153:2152-2163, 2012
35. Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB: Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 64:106-110, 1987
36. Gromada J, Franklin I, Wollheim CB: Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28:84-116, 2007
37. Mitrakou A, Kelley D, Mokan M, Veneman T, Pangburn T, Reilly J, Gerich J: Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med 326:22-29, 1992
38. Ferrannini E, Muscelli E, Natali A, Gabriel R, Mitrakou A, Flyvbjerg A, Golay A, Hojlund K: Association of fasting glucagon and proinsulin concentrations with insulin resistance. Int J Obes Relat Metab Disord 50:2342-2347, 2007
39. He S, Wang D, Lu Y, Chen Y, Jin X, Wang C, Zhao J, Ren Y, Wang L, Li H, Cheng J: Increasing glucagon secretion could antagonize the action of exogenous insulin for glycemic control in streptozocin-induced diabetic rhesus monkeys. Exp Biol Med (Maywood ) 238:385-391, 2013
40. Ahren B: Beta- and alpha-cell dysfunction in subjects developing impaired glucose tolerance: outcome of a 12-year prospective study in postmenopausal Caucasian women. Diabetes 58:726-731, 2009
41. Chen L, Klein T, Leung PS: Effects of combining linagliptin treatment with BI-38335, a novel SGLT2 inhibitor, on pancreatic islet function and inflammation in db/db mice. Curr Mol Med 12:995-1004, 2012
42. Christensen M, Bagger JI, Vilsboll T, Knop FK: The alpha-cell as target for type 2 diabetes therapy. Rev Diabet Stud 8:369-381, 2011
43. Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, Donath MY: Sulfonylurea induced beta-cell apoptosis in cultured human islets. J Clin Endocrinol Metab 90:501-506, 2005
44. Forst T, Dworak M, Berndt-Zipfel C, Loffler A, Klamp I, Mitry M, Pfutzner A: Effect of vildagliptin compared to glimepiride on postprandial proinsulin processing in the beta cell of patients with type 2 diabetes mellitus. Diabetes Obes Metab 15:576-579, 2013
45. Landstedt-Hallin L, Arner P, Lins PE, Bolinder J, Olsen H, Groop L: The role of sulphonylurea in combination therapy assessed in a trial of sulphonylurea withdrawal. Diabet Med 16:827-834, 1999
46. Bohannon NV, Lorenzi M, Grodsky GM, Karam JH: Stimulatory effects of tolbutamide infusion on plasma glucagon in insulin-dependent diabetic subjects. J Clin Endocrinol Metab 54:459-462, 1982
47. Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB: Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54:1808-1815, 2005
48. Forst T, Anastassiadis E, Diessel S, Loffler A, Pfutzner A: Effect of linagliptin compared to glimepiride on postprandial glucose metabolism, islet cell function, and vascular function parameters in patients with type 2 diabetes mellitus on ongoing metformin treatment. Diabetes Metab Res Rev 2014
49. Ahren B, Schweizer A, Dejager S, Dunning BE, Nilsson PM, Persson M, Foley JE: Vildagliptin enhances islet responsiveness to both hyper- and hypoglycemia in patients with type 2 diabetes. J Clin Endocrinol Metab 94:1236-1243, 2009
50. Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ: Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 124:499-508, 2014
51. Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, Xiong J, Perez Z, Norton L, Abdul-Ghani MA, DeFronzo RA: Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 124:509-514, 2014




