A promising approach that could be a game-changer for patients with diabetes.
Since its discovery and isolation, exogenous insulin has resulted dramatically change in the prognosis for patients with diabetes. Nowadays, novel insulin analogues have improved pharmacokinetic profiles mirroring endogenous basal and prandial insulin secretion more closely. However, despite advances in insulin formulations and in closed loop systems combined with advancedcontinuous glucose-monitoring systems and external insulin infusion pumps, glucose control still remains a challenge. Patients with diabetes do not achieve their glycemic targets and hypoglycemia continues to be the major hurdle for intensification of insulin therapy.
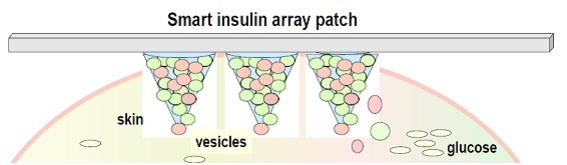
Figure 1 - The smart insulin patch (microneedle array patch) (simplified representation)
So far, the early-stage preclinical pipeline for glucose-responsive insulin (smart insulin) is fairly crowded, whereas the majority of the current advanced candidates have not progressed beyond preclinical development.
This is unfortunate; because a successful "smart insulin" in glucose-responsive "closed-loop" insulin delivery system that is able to deliver insulin in response to elevated blood glucose would provide optimized glucose control with minimal patient effort and potential improvement in quality of life for patients with diabetes.
A working group of North Carolina State University (UNC) has developed a promising delivery system so called “smart insulin patch” (array of tiny needles) (see Figure 1) which could be placed anywhere on the body to detect and deliver insulin according to changes in the glucose concentrations. Therefore, a perfect glucose-responsive insulin delivery system might be a game-changer for patients with diabetes.
How does the joint effort between diabetologists and biomedical engineers work?
Smart insulins behave like alarm call centers, sensing increases in glucose levels with subsequent release of insulin. These approaches generally integrate a glucose sensing or conversion module and a sensing/conversion activated insulin releasing module. Three classic strategies are often utilized comprising different glucose-sensing moieties: glucose oxidase (GOx) [[1]], glucose-binding protein (GBP, i.e. Con A) [[2], [3]] and glucose-binding molecules (GBM, i.e. phenylboronic acid (PBA)) for achieving glucose triggers [[4]]. A variety of formulations, such as bulk hydrogels, microgels, emulsion based nanoparticles, and self-assembled vesicles have been developed to respond to glucose concentration changes by structural transformations such as swelling, shrinking, degrading or dissociating in order to promote the release of insulin [[5], [6], [7], [8]].
Some innovative strategies in combination with smart insulins
The working group of UNC creates “glucose response cells” to realize a combination of fast responsiveness and long-term persistence.
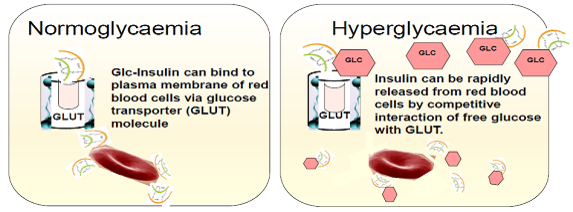
Figure 2 - Glucose responsive red blood cells (simplified representation)
An innovative strategy to overcome the use of synthetic materials has been generated by the integration of red blood cells (RBC) and glucose derivative-modified insulin (Glc-Insulin). After being conjugated with glucosamine, insulin can efficiently bind to red blood cells by interacting with glucose transporter (GLUT) on plasma membranes. The reversible binding between glucose derivative-modified insulin (Glc-Insulin) and GLUT can enable a fast insulin release from the cellular carrier in the setting of hyperglycaemia (illustrated in Figure 2). The delivery vehicle can be further simplified utilizing injectable polymeric nanocarriers coated with red blood cell membrane and loaded with Glc-Insulin [8]. This approach could be simply integrated with a microneedle-array patch.
There remains the challenge to demonstrate a desirable insulin delivery system, which combines ease of use, high drug capacity loading for longer use, fast responsiveness and excellent biocompatibility.
Most glucose-responsive formulations that incorporated glucose oxidase (GOx) involve pH sensitive materials based on the enzymatic oxidation of glucose to gluconic acid. Local decline of pH associated with increasing blood glucose levels promote the insulin release through either by degradation or protonation [7]. Nevertheless, these systems are limited because of the challenge of rapidly switching the physiological pH in vivo [[9]].
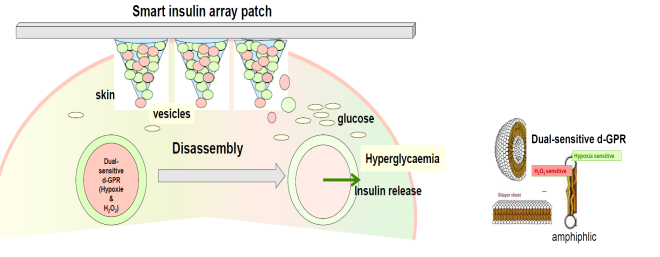
Figure 3 – Glucose-responsive insulin delivery system using hypoxia and H2O2–responsive polymersome vesicles loading with microneedle-array patches (simplified representation).
The working group of UNC has presented a closed-loop, glucose-responsive insulin delivery system by integrating hydrogen peroxide (H2O2)–responsive polymeric vesicles with a transcutaneous microneedle array patch to sense glucose and release insulin across the skin layer. Vesicles are microscopic vesicles that enclose a volume with a molecularly thin membrane. The membranes are generally self-directed assemblies of amphiphilic molecules with dual hydrophilic-hydrophobic characteristics. Utilizing polymeric vesicles, water-soluble insulin has been encapsulated into the inner cavity which has a high loading capacity. In the setting of hyperglycaemia glucose will diffuse across the membrane and interact with GOx in the cavity, which leads to the oxidation of glucose to gluconic acid, simultaneously generating H2O2. By virtue of the generated H2O2, the copolymer becomes watersoluble with leading to disassembly of the polymeric vesicles and the subsequent release of the preloaded insulin. This formulation demonstrated both in vitro and in vivo glucose-mediated disassembly, releasing the encapsulated insulin under hyperglycaemic conditions with rapid responsiveness, without insulin release in normoglycaemic conditions [[10]].
As rapid H2O2 accumulation may lead to local inflammation, the active glucose-responsive insulin delivery system has been further exploited utilizing polymersome vesicles sensitive to both hypoxia and H2O2 (smart insulin patch). These polymersome-based vesicles (dual sensitive d-GRP) were able to eliminate excess H2O2 and to reduce tissue inflammation while maintaining the activity of GOX (Figure 3). Furthermore, these vesicles can be integrated within a cross-linked hyaluronic acid-based (natural substance) microneedle–array patch to achieve convenient, painless and continuous administration of insulin with high biocompatibility. In vivo experiments indicated that this enhanced smart insulin patch was highly effective in providing tight blood glucose regulation in diabetic mice and showed minimal side effects regarding inflammation. For potential translation of this microneedle array patch-based formulation in to clinical use, efforts associated with further enhancement of loading capability and bioavailability are expected [[11]].
Outlook
These promising innovative strategies will require further investigations in early clinical development. Long-term goal is to develop a smart insulin patch that patients would only have to change every few days. If these patches will work in patients with diabetes it might prove to be a game changer in insulin treatment of patients with diabetes mellitus.
References
[1] Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. PNAS. 2015; Vol 112, No.27:8260-8265.
[2] Liu F, Song SC, Mix D, Baudys M, Kim SW. Glucose induced release of glycosylpoly(etylen glycol) insulin bound to a soluble conjugate of concanavalin A. Bioconjug Chem:1997;8(5):664-672.
[3] Baudys M et al. Glycolysated insulins. J Control Release.1995;36(1-2):151-157.
[4] Chou H-C, Webber MJ, Tang , Lin AB, Thapa LS, Deng D, Truong JV, Cortinas AB, Langer R, AndersonDG. Glucose-responsive insulin activity by covalent modification with aliphatic phenylbaronic acid conjugates. PNAS.2015; Vol 112, No.8: 2401-2406.
[5] Gu Z, Dang TT, Ma M et al. Glucose-responsive microgels integrated with enzyme nano-capsules for closed-loop insulin delivery. ACS Nano. 2013; 7(8):6758-6766.
[6] Gu Z et al. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano. 2013; 7(5):4194-4201.
[7] Podual K, Doyle FJ, Peppas N. Glucose-sensitivity of glucose oxidase-containing cationic copolymer hydrogels having poly(ethlene glycol) grafts. 2000. J Control Release 67(1):9-17.
[8] Wang C, Ye Y, Sun W, Yu J, Wang J, Lawrence DS, Buse JB, Gu Z. Red Blood Cells for Glucose-Responsive Insuln Delivery. Advanced Materials. 2017. DOI: 10.1002/adma.201606617.
[9] Mo R, Jiang T, Di J, Tai W, Gu Z. Emerging Micro- and Nanotechnologiy Based Synthetic Approaches for Insulin Delivery. Chem. Soc. Rev. 2014; 43<.3595-3629.
[10] Hu X, Yu J, Qian C, Lu Y, Kahkoska AR, Xie Z, Jing X, Buse JB, Gu Z. H2O2 Response Vesicles integrated with Transcutaneous Patches for Glucose –Mediated Insulin Delivery. ACS Nano. 2017; 11, 613-620.
DOI: 10.1021/acs.nano.6b06892.
[11] Yu J, Qian C, Zhang Y Cui Z, Zhu Y, Shen Q, Ligler FS, Buse JB, Gu Z. Hypoxia and H2O2 Dual-Sensitive Vesicles for Enhanced Glucose-Responsive Insulin Delivery. ACS.2017: DOI:10.1021/acs.nanolett6b03848.




